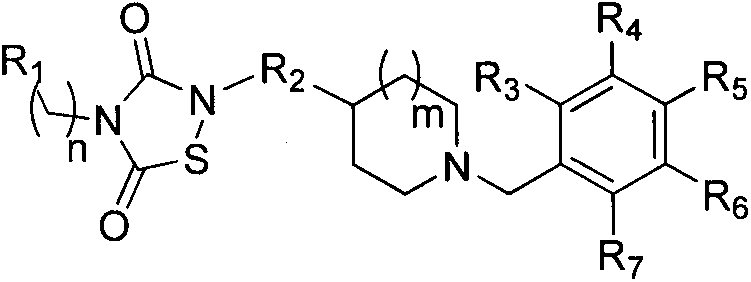
1,2,4-thiadiazole-3,5-dione derivatives, and pharmaceutical composition and application thereof
A compound, C2-C8 technology, used in drug combinations, anti-tumor drugs, cardiovascular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
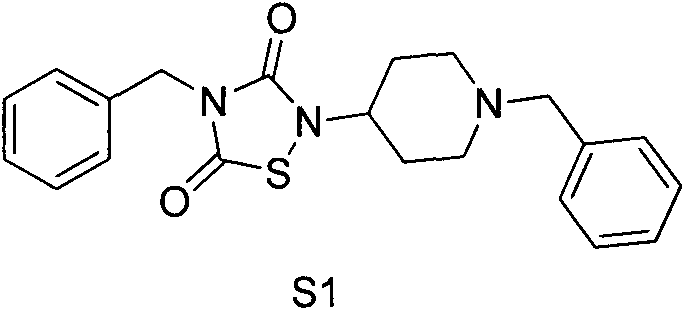
[0211] Embodiment 1: the synthesis of compound S1
[0212] synthetic route:
[0213]
[0214] Synthesis of step 1 benzyl thioisocyanate
[0215] Benzylamine (9.63g, 90mmol) and triethylamine (18.18g, 180mmol) were mixed and dissolved in dichloromethane, and carbon disulfide (9g, 120mmol) was added dropwise under stirring in an ice bath, and stirred for 1h under ice bath after dropping, Then add triphosgene (8.82g, 0.30mol), stir at room temperature for 4h, wash with saturated aqueous sodium bicarbonate solution, dry the dichloromethane layer with anhydrous sodium sulfate, perform column chromatography, and elute with petroleum ether to obtain a colorless and transparent solution 11.18 g, yield 83.2%.
[0216] Synthesis of Step 2 Raw Material A
[0217] Triphosgene (BTC, 1.78g, 6mmol) was dissolved in chloroform, and a mixed solution of DIPEA (4.65g, 36mmol) and N-Boc piperidin-4-amine (4.0g, 20mmol) was added dropwise under ice cooling. Stir overnight in an ice bath, w...
Embodiment 2
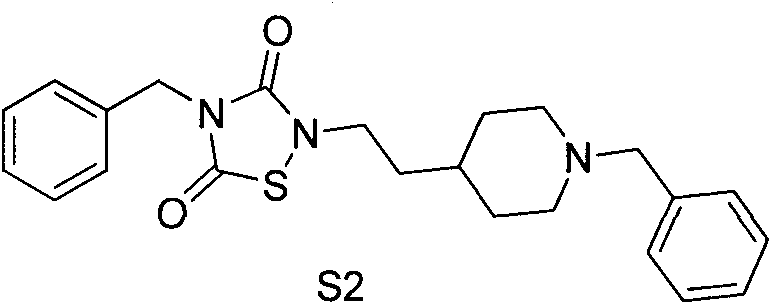
[0224] Embodiment 2: the synthesis of compound S2
[0225] synthetic route:
[0226]
[0227] Synthesis of Step 1 Raw Material C
[0228] Dissolve piperidine-4-ethanol (1.8g, 14mmol) and DIPEA (3.6g, 28mmol) in 20ml of anhydrous acetonitrile, add benzyl bromide (2.38g, 14mmol) dropwise, and heat to reflux overnight. TLC monitors the raw material Disappeared, reaction liquid column chromatography, petroleum ether: ethyl acetate = 1:1, 2.3g of transparent oil was obtained, yield 75.01%, ESI-MS: 220.1 [M+H] + ;
[0229] Synthesis of Step 2 Intermediate D
[0230] Intermediate C (2.19g, 10mmol) was dissolved in 5ml of DMF, carbon tetrabromide (6.54g, 20mmol) was added, triphenylphosphine (5.24g, 20mmol) was added dropwise under ice-cooling, and stirring was completed under ice-bath After 20 min, stirring at room temperature overnight, column chromatography petroleum ether: ethyl acetate = 8:1 ~ 6:1, 1.5 g of yellow transparent oil was obtained with a yield of 53.38%.
[0...
Embodiment 3
[0239] Embodiment 3: the synthesis of compound S3
[0240] synthetic route:
[0241]
[0242] Synthesis of Step 1 Intermediate F
[0243] Dissolve piperidine-4-propanol (2.86g, 20mmol) and DIPEA (5.16g, 40mmol) in 20ml of anhydrous acetonitrile, add benzyl bromide (6.8g, 40mmol) dropwise, heat to reflux overnight, monitor by TLC The raw material disappeared, the reaction liquid column chromatography, petroleum ether: ethyl acetate = 1:1, 3.5 g of transparent oil was obtained, the yield was 75.11%, ESI-MS: 234.2 [M+H] + ;
[0244] Synthesis of Step 2 Intermediate G
[0245] Intermediate F (2.33g, 10mmol) was dissolved in 5ml of DMF, carbon tetrabromide (6.54g, 20mmol) was added, triphenylphosphine (5.24g, 20mmol) was added dropwise under ice-cooling, and stirring was completed under ice-bath After 20 min, stirring at room temperature overnight, column chromatography petroleum ether: ethyl acetate = 8:1 ~ 6:1, 1.83 g of yellow transparent oil was obtained with a yield of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com