Application of eukaryotic peptide chain release factor 3b fragment 36 (eRF3b-36) in treatment of liver injury
A technology of erf3b-36 and release factors, which is applied in the field of biopharmaceuticals, can solve the problems of limited fat elimination, inability to remove liver fat, liver cell damage, etc., and achieve the effects of less toxic side effects, good preventive and therapeutic effects, and reduced content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] eRF3b injection in Example 1 (human eRF3b fragment (eRF3b-36) solution):
[0059] Human eRF3b fragment (eRF3b-36) lyophilized powder 2mg
[0060] Sterile normal saline 1ml
[0061] Pipette repeatedly with a pipette until the lyophilized powder is completely dissolved, aliquot into 100ul tubes, and store at -20°C. Before use, dilute with sterile saline according to the needs of the experiment.
[0062] Preparation of 4% paraformaldehyde solution:
[0063]
[0064] Stir the above components fully at 50°C until they are completely dissolved, adjust the pH to 7.4, and add double distilled water to make up to 1L.
[0065] Male Balb / c inbred mice (6-8 weeks old) were purchased from the Experimental Animal Center of Hebei Medical University.
[0066] Male SD rats (180-200 g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0067] Preparation of PI staining solution: 129.6ml of normal saline, 10mg of PI, 2mg of RNase, 1.0% by volume o...
Embodiment 2
[0076] The eRF3b injection in embodiment 2:
[0077] Human eRF3b fragment (eRF3b-36) lyophilized powder 5mg
[0078] Sterile normal saline 2ml
[0079] Pipette repeatedly with the pipette until the lyophilized powder is completely dissolved, and store at -20°C. Dispense 500 μl per tube into 1.5ml EP tubes and dilute with sterile saline to the administration concentration before use.
[0080] The acute liver injury induced by Con A in Example 1 is acute immune liver injury.
[0081] The physiological saline in the following examples is sterile physiological saline.
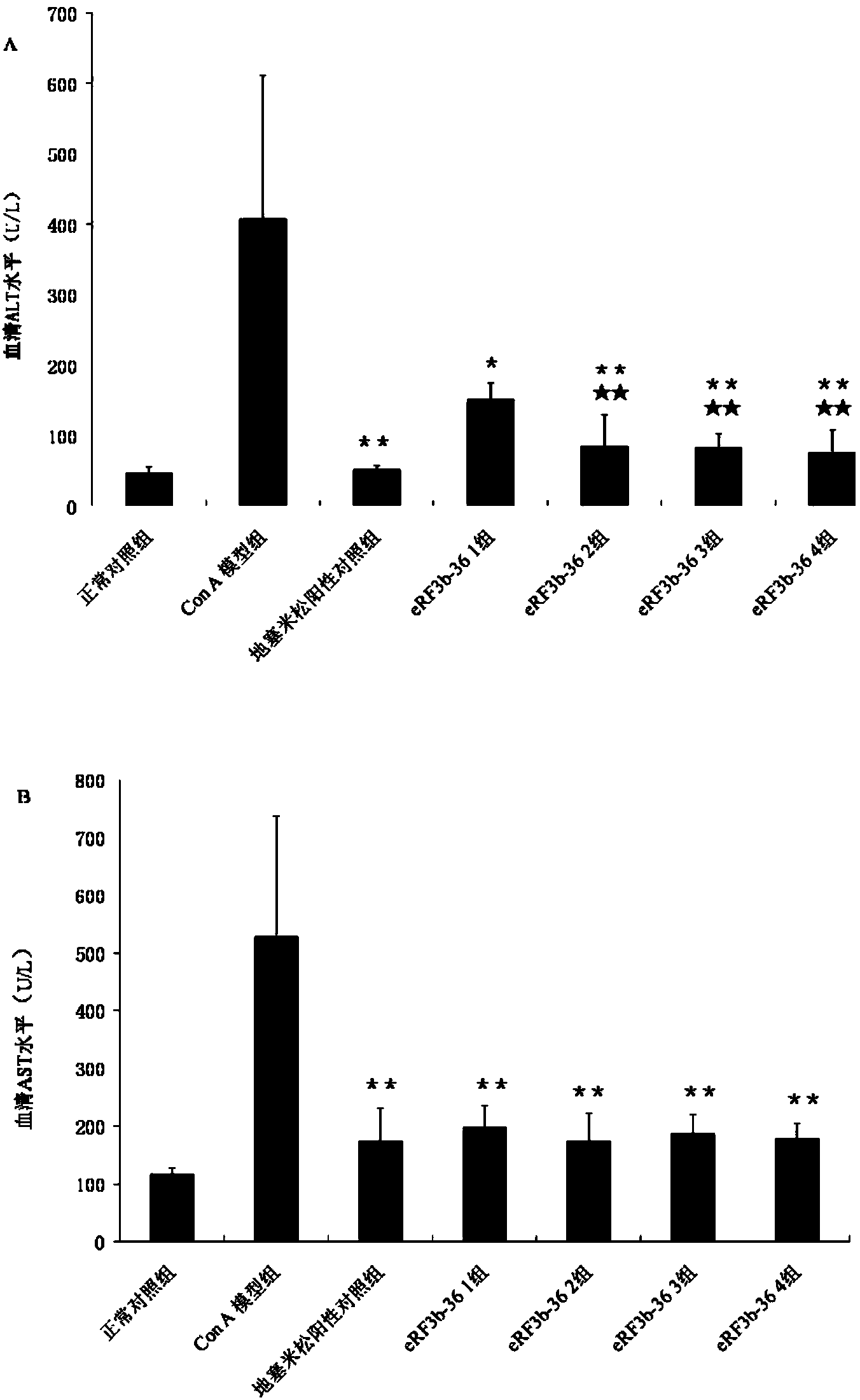
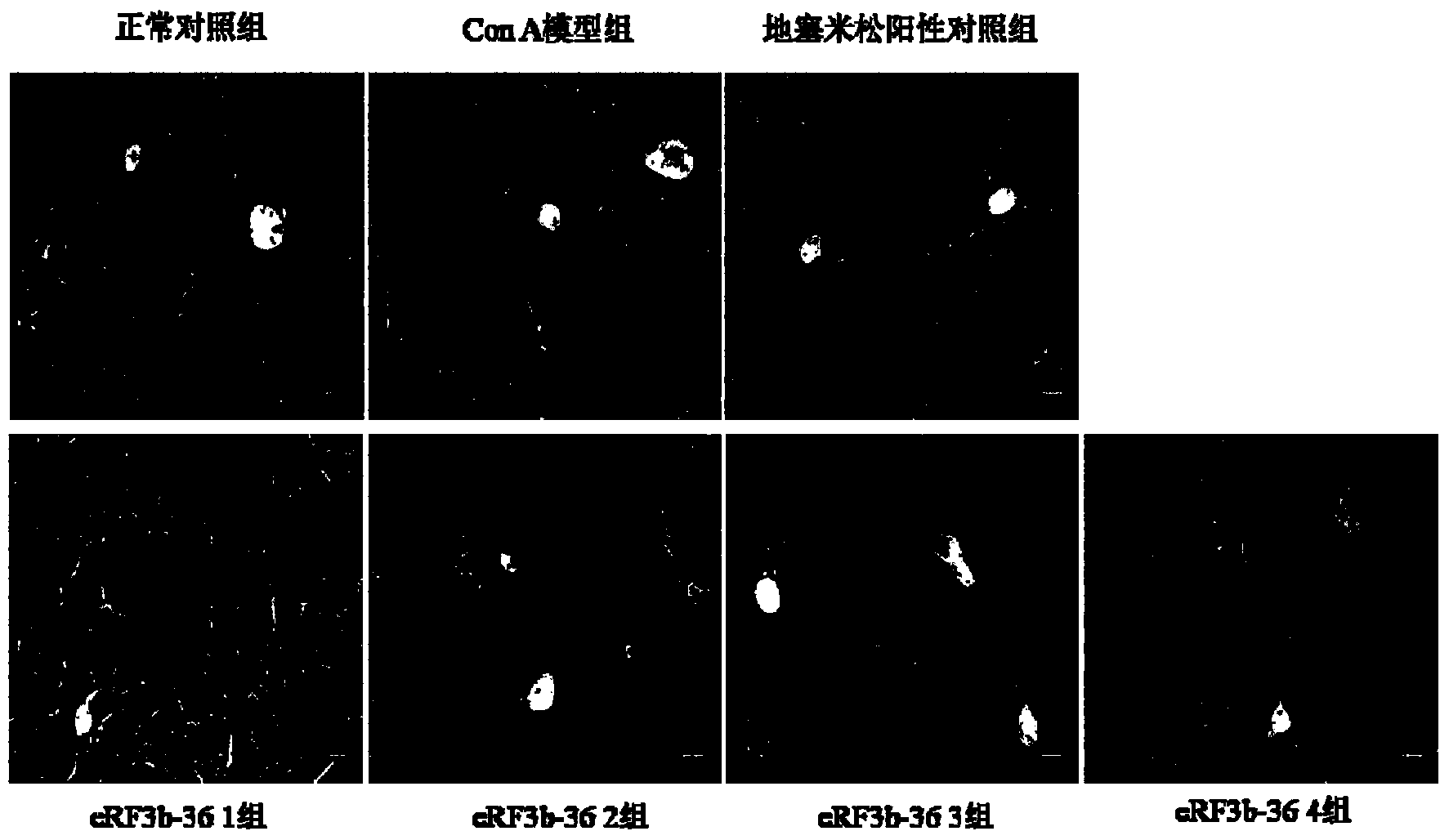
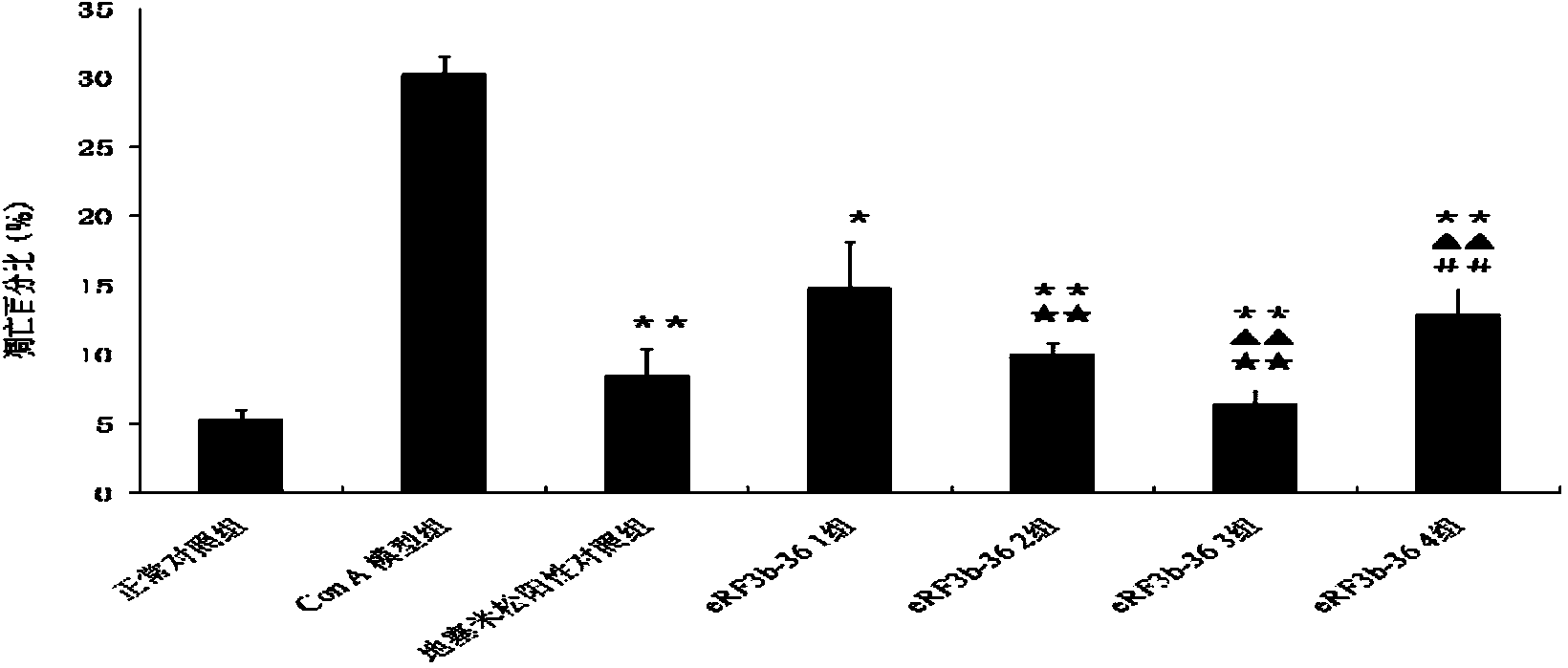
[0082] Example 1. Application of human eRF3b fragment (eRF3b-36) in the treatment of acute liver injury
[0083] 1. Prevention experiment
[0084] (1) Experimental grouping
[0085] 56 male Balb / c inbred mice (6-8 weeks old) were randomly divided into 7 groups, namely normal control group, ConA model group, dexamethasone positive control group, eRF3b-361 group, eRF3b-362 group , eRF3b-363 group and eRF3b-364...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com