New synthesis method for aromatic alpha-hydroxy ketone compounds
A synthetic method and technology of hydroxy ketones, applied in the field of ultraviolet light curing photoinitiators, can solve the problems of uncompetitiveness in industrial production, difficulties in equipment maintenance, and difficulties in the treatment of three wastes, and achieve low overall cost of raw materials, low equipment requirements, The effect of saving equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
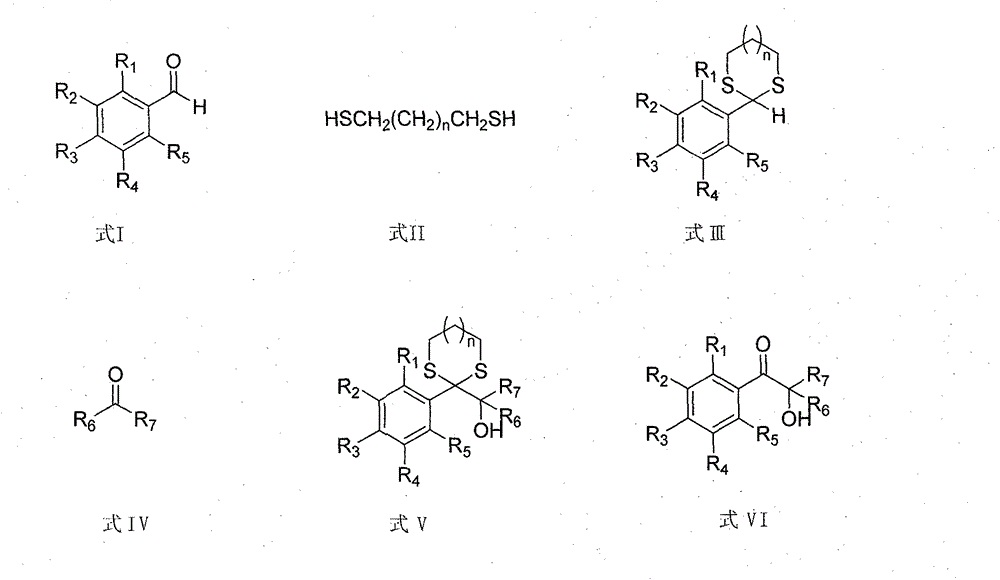
[0060] 1.1 Add 106g benzaldehyde (1mol), 113g propanedithiol (1.05mol), 2L acetonitrile to a 5000ml four-neck flask, add catalyst Al2O3-SO3H (50g, 0.15mol H+), stir at room temperature until the reaction is complete (GC tracking ). After shrinking the reaction system to dryness, add dichloromethane to dissolve, filter, wash the catalyst with a small amount of dichloromethane, dry and activate it, and use it mechanically. The organic phase is sequentially filled with 2L of 10% sodium hydroxide solution, 2L of water, and 1L of saturated brine. After washing, drying with anhydrous sodium sulfate and shrinking to dryness, the product 2-phenyl-1,3-dithiane was obtained with a melting point of 68-70° C. and a yield of 98%.
[0061] 1.2 Add 0.5mol 2-phenyl-1,3-dithiane and 1.5L dried tetrahydrofuran to the bottle under nitrogen protection, stir, cool down to -30°C, add 218ml of n-butyl lithium solution (2.3M) dropwise, Keep warm at -30°C and stir for 1 hour, add 54 g of cyclohexanon...
Embodiment 2
[0064] 2.1 Add 106g benzaldehyde (1mol), 113g propanedithiol (1.05mol), 2L acetonitrile to a 5000ml bottle, add catalyst Al2O3-SO3H (50g, 0.15mol H+), stir at room temperature until the reaction is complete (GC tracking). After shrinking the reaction system to dryness, add dichloromethane to dissolve, filter, wash the catalyst with a small amount of dichloromethane, dry and activate it, and use it mechanically. The organic phase is washed successively with 2L of 10% sodium hydroxide solution, 2L of water and 1L of saturated brine , dried over anhydrous sodium sulfate, and shrunk to give the product 2-phenyl-1, 3-dithiane. The melting point is 68-70°C, and the yield is 98%.
[0065] 2.2 Add 0.5mol 2-phenyl-1,3-dithiane and 1.5L dried tetrahydrofuran to the bottle under nitrogen protection, stir, cool down to -30°C, add 218ml of n-butyl lithium solution (2.3M) dropwise, Keep warm at -30°C and stir for 1 hour, add 32g of acetone to the system, after the addition is complete, the...
Embodiment 3
[0068] 3.1 Add 106g benzaldehyde (1mol), 113g propanedithiol (1.05mol), 2L acetonitrile to a 5000ml bottle, add catalyst Al2O3-SO3H (50g, 0.15mol H+), stir at room temperature until the reaction is complete (GC tracking). After shrinking the reaction system to dryness, add dichloromethane to dissolve, filter, wash the catalyst with a small amount of dichloromethane, dry and activate it, and use it mechanically. The organic phase is washed successively with 2L of 10% sodium hydroxide solution, 2L of water and 1L of saturated brine , dried over anhydrous sodium sulfate, and shrunk to give the product 2-phenyl-1, 3-dithiane. The melting point is 68-70°C, and the yield is 98%.
[0069] 3.2 Add 0.5mol 2-phenyl-1,3-dithiane and 1.5L dried tetrahydrofuran to the bottle under nitrogen protection, stir, cool down to -30°C, add 218ml of n-butyl lithium solution (2.3M) dropwise, Keep warm at -30°C and stir for 1h, add 46g of cyclopentanone to the system, after the addition is complete, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com