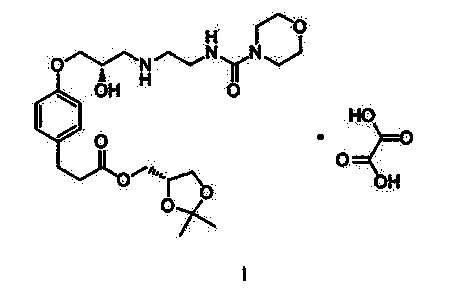
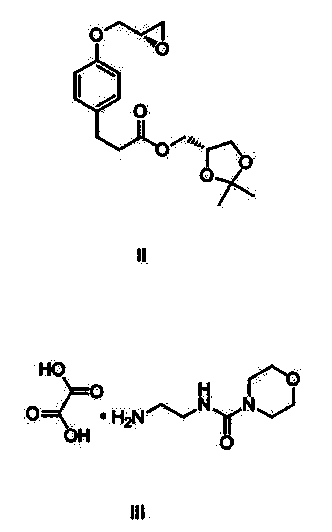
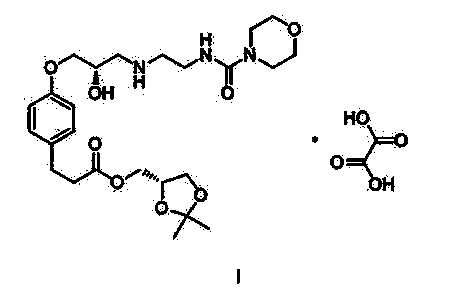
Method for preparing Landiolol oxalate
A technology of devolol oxalate and alkali metal carbonate, applied in the directions of carboxylate preparation, organic chemistry, etc., can solve the problems of difficult to use pharmaceutical injection preparations, low product yield, poor quality, etc., and avoid alcohol Solution, simple operation, improved productivity and product quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of compound I
[0031] Add 1565.5g of compound III and 3000g of water into the reaction flask, stir and heat up to t 内 =45°C, dissolve clear, use 1190g of 40% sodium hydroxide (475g of sodium hydroxide dissolved in 715g of water) to adjust the pH to 10-11, control the temperature below 50°C, a large amount of yellow solid precipitates, cool down to 30-35°C in an ice bath , add 500g of compound Ⅱ, 3000g of N,N-dimethylformamide, keep the reaction at 30~35℃ for 3~4h, after TLC confirms that the reaction of compound Ⅱ is complete, filter, extract the filtrate with 800mL×3 isopropyl ether, collect water layer, extracted with 3000 mL×4 ethyl acetate, combined the organic layers, and washed with 5000 mL×3 saturated brine. Collect the organic layer, add 2000g of anhydrous sodium sulfate and 100g of activated carbon, stir and dry for 2-3 hours to decolorize. Filtrate, evaporate the filtrate to dryness at 60°C under reduced pressure, add 5.0kg o...
Embodiment 2
[0032] Embodiment 2, the preparation of compound I
[0033] Add 1956.9g of compound III and 5000g of water into the reaction flask, stir and heat up to t内 =45°C, dissolve clear, use 1313g of 40% sodium hydroxide (525g of sodium hydroxide dissolved in 788g of water) to adjust the pH to 10-11, control the temperature below 50°C, a large amount of yellow solid precipitates, cool down to 40-45°C in an ice bath , add 500g of compound II, 5000g of dimethyl sulfoxide, and incubate at 40-45°C for 3-4h. After TLC confirms that the reaction of compound II is complete, filter and extract the filtrate with 800mL×3 isopropyl ether, collect the aqueous layer, and wash with 3000mL× 4 Extract with ethyl acetate, combine the organic layers, and wash with 5000 mL×3 saturated brine. Collect the organic layer, add 2000g of anhydrous sodium sulfate and 100g of activated carbon, stir and dry for 2-3 hours to decolorize. Filtrate, evaporate the filtrate to dryness under reduced pressure at 60°C, ...
Embodiment 3
[0034] Embodiment 3, the preparation of compound I
[0035] Add 1174.2g of compound III and 1500g of water into the reaction flask, stir and heat up to t 内 =45°C, dissolve clear, use 40% sodium hydroxide 1063g (425g sodium hydroxide dissolved in 638g water) to adjust the pH to 10-11, control the temperature below 50°C, a large amount of yellow solid precipitates, at 45-50°C, add the compound Ⅱ 500g, N,N-dimethylacetamide 1500g, keep the reaction at 45~50℃ for 3~4h, TLC confirms that the reaction of compound Ⅱ is complete, filter, and extract the filtrate with 800mL×3 isopropyl ether, collect the water layer, and use Extract with 3000 mL×4 ethyl acetate, combine the organic layers, and wash with 5000 mL×3 saturated brine. Collect the organic layer, add 2000g of anhydrous sodium sulfate and 100g of activated carbon, stir and dry for 2-3 hours to decolorize. Filtrate, evaporate the filtrate to dryness under reduced pressure at 60°C, add 6.5kg of absolute ethanol to the residu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com