Synthetic method of 1-nitro-3,4:9,10-perylene tetracarboxylate and 1,6(7)-dinitro-3,4:9,10-perylene tetracarboxylate
A technology of perylene tetracarboxylate and a synthesis method, which is applied in the field of organic synthesis, can solve the problems of low yield, non-single product, difficult to control and the like, and achieves the effects of high yield, single product and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
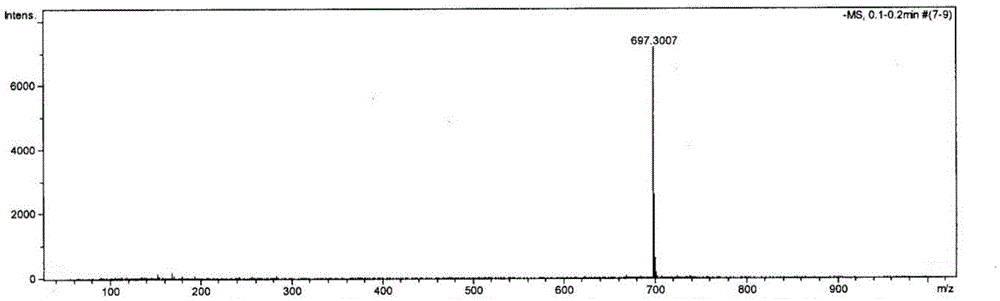
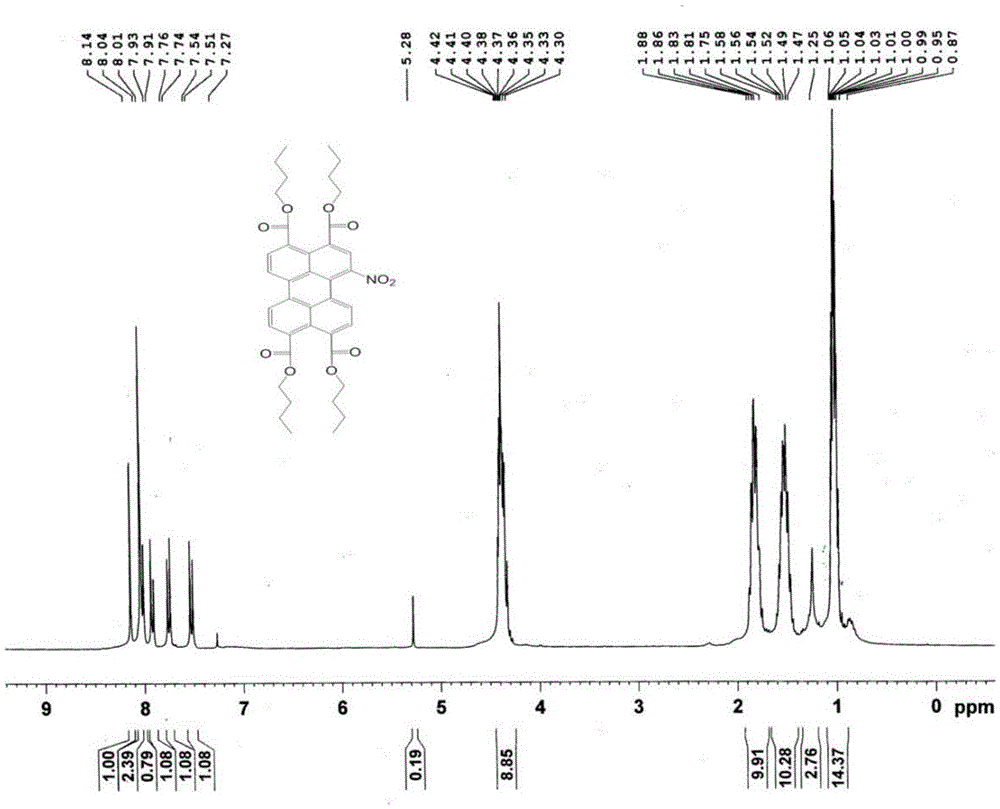
[0031] In a 50mL round bottom flask, add 3,4:9,10-n-butyl perylenetetracarboxylate (200mg, 0.3mmol) and 20mL of dichloromethane, ultrasonically shake to dissolve, and slowly drop into the reaction system under stirring at room temperature Add 0.5mL fuming nitric acid, follow the reaction by TLC, and react for about 1 hour. When 1,6(7)-dinitro-3,4:9,10-perylenetetracarboxylate is identified by TLC, the reaction is stopped, and the The reaction solution was transferred to a separatory funnel, washed repeatedly until the water layer became neutral, the organic layer was separated, dried over anhydrous sodium sulfate, the solvent was removed by rotary evaporation, and vacuum-dried at 70°C for 5 hours. The crude product was separated and purified by silica gel column chromatography (dichloromethane:petroleum ether=2:1) to obtain 190 mg of n-butyl 1-nitro-3,4:9,10-perylenetetracarboxylate with a purity of 99%. The yield was 91%. figure 1 For the mass spectrogram of the compound p...
Embodiment 2
[0033] In a 50mL round bottom flask, add 3,4:9,10-n-butyl perylenetetracarboxylate (200mg, 0.3mmol) and 20mL of chloroform, ultrasonically shake to dissolve, and slowly drop into the reaction system under stirring at room temperature Add 0.5mL fuming nitric acid, follow the reaction by TLC, and react for about 1 hour. When 1,6(7)-dinitro-3,4:9,10-perylenetetracarboxylate is identified by TLC, the reaction is stopped, and the The reaction solution was transferred to a separatory funnel, washed repeatedly until the water layer became neutral, the organic layer was separated, dried over anhydrous sodium sulfate, the solvent was removed by rotary evaporation, and vacuum-dried at 70°C for 5 hours. The crude product was separated and purified by silica gel column chromatography (dichloromethane:petroleum ether=2:1) to obtain 192 mg of n-butyl 1-nitro-3,4:9,10-perylenetetracarboxylate with a purity of 99%. The yield was 92%.
Embodiment 3
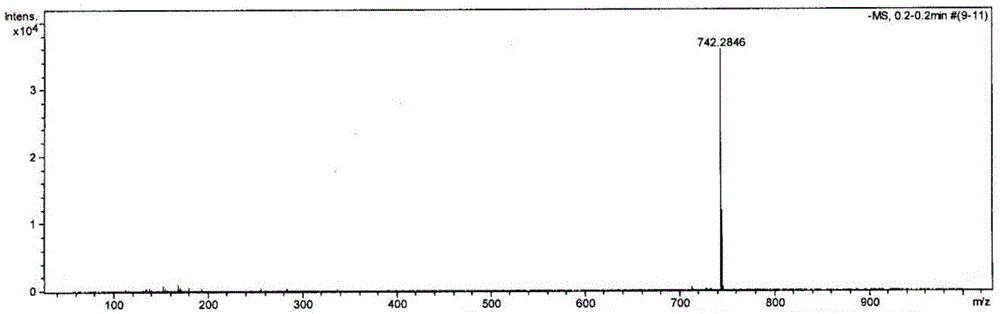
[0035] In a 50mL round bottom flask, add 3,4:9,10-n-butyl perylenetetracarboxylate (200mg, 0.3mmol) and 20mL of dichloromethane, ultrasonically shake to dissolve, and slowly drop into the reaction system under stirring at room temperature Add 1.5mL fuming nitric acid, follow the reaction by TLC, and react for about 5 hours. When 1-nitro-3,4:9,10-perylenetetracarboxylate cannot be identified by TLC, stop the reaction, and transfer the reaction solution to the liquid funnel, washed repeatedly until the water layer was neutral, separated the organic layer, dried over anhydrous sodium sulfate, rotary evaporated to remove the solvent, and dried under vacuum at 70°C for 5h. The crude product was separated and purified by silica gel column chromatography (dichloromethane:petroleum ether=2:1) to obtain 200 mg of n-butyl 1,6(7)-dinitro-3,4:9,10-perylenetetracarboxylate , with a purity of 99% and a yield of 90%. image 3 For the mass spectrogram of the compound prepared in this embod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com