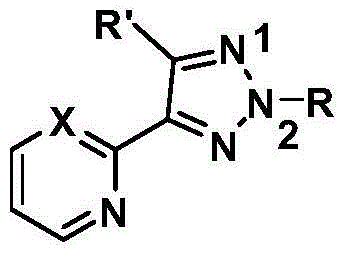
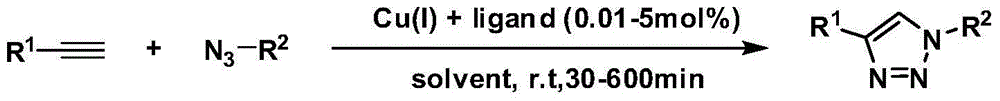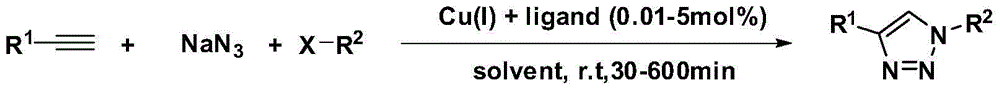N2-substituted 1,2,3-triazole derivative for Cu (I) ligand as well as preparation method and application of N2-substituted 1,2,3-triazole derivative
A technology of triazole derivatives and ligands, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., can solve problems such as low reaction yield, difficult synthesis, and application limitations , to achieve the effects of high product yield, strong substrate adaptability, and small catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Ligand 1 Synthesis:
[0028]
[0029] 4-(2-pyridine)-NH-1,2,3-triazole (0.84g, 10mmol), benzyl bromide (1.88g, 11mmol), potassium carbonate (99mg, 1mmol), Potassium iodide (0.23g, 2mmol), DMF (40mL) was used as the reaction solvent, stirred at room temperature for 1-2h, the reaction was complete, and the reaction process was monitored by TLC. After the reaction was completed, it was extracted with ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration and concentration to obtain the crude product was separated and purified by thin-layer silica gel column chromatography to obtain N1 substituted product and N2 substituted product respectively, wherein the N2 substituted product was a white solid which was Ligand 1, with 2.13g and a yield of 60% . The structure of the compound is characterized as follows: 1H NMR (400MHz, CDCl3) δ8.53(s, 1H), 8.19(d, J=7.9Hz, 1H), 8.05(s, 1H), 7.78-7.75(m, 1H) ,7.37(s,1H),7.22–7.19(m,5H),5.58(s,2H...
Embodiment 2
[0031] Ligand 2 Synthesis:
[0032]
[0033] Add 5-phenyl-4-(2-pyridine)-NH-1,2,3-triazole (0.84g, 10mmol), benzyl bromide (1.88g, 11mmol), potassium carbonate ( 99mg, 1mmol), potassium iodide (0.23g, 2mmol), DMF (40mL) was used as the reaction solvent, stirred at room temperature for 1-2h, the reaction was complete, and the reaction process was monitored by TLC. After the reaction was completed, it was extracted with ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration and concentration to obtain a crude product was separated and purified by thin-layer silica gel column chromatography to obtain a white solid which is Ligand 2, 2.00 g, with a yield of 74%. The structure of the compound is characterized as follows: 1H NMR (400MHz, CDCl3) δ8.45 (s, 1H), 7.75 (d, J=3.6Hz, 1H), 7.64-7.62 (m, 2H), 7.47-7.40 (m, 3H),7.33–7.15(m,4H),7.12(s,1H),7.03(s,2H),5.45(s,2H).
Embodiment 3
[0035] Ligand 3 Synthesis:
[0036]
[0037]Add 5-phenyl-4-(2-pyridine)-NH-1,2,3-triazole (1.00g, 10mmol), iodobenzene (1.02g, 11mmol), potassium carbonate ( 100mg, 1mmol), cuprous iodide (59mg, 0.1mmol), L-proline (50mg, 0.1mmol), with DMF (40mL) as the reaction solvent, heated to 80°C under the protection of argon and stirred for 1~2h The reaction was complete, and the reaction process was monitored by TLC. After the reaction was completed, it was extracted with ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 After drying, filtration and concentration, the crude product was separated and purified by thin-layer silica gel column chromatography to obtain a white solid which was Ligand 3, 1.76 g, with a yield of 87%. The structural characterization of the compound is as follows: 1H NMR (400MHz, CDCl3) δ8.69(s,1H),7.67–7.63(m,6H),7.38(s,4H),7.26(s,2H),6.88(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com