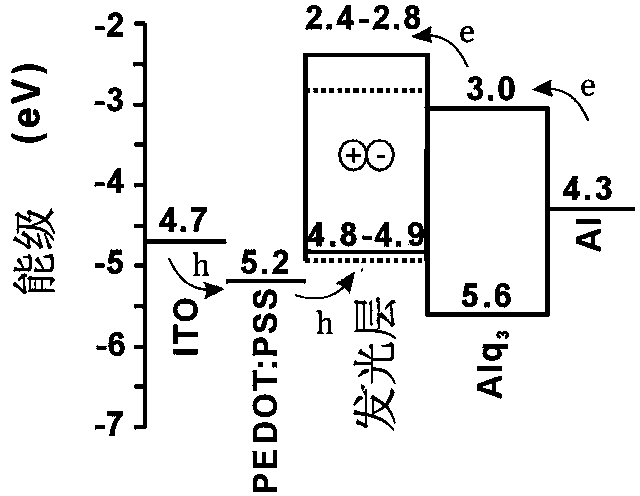
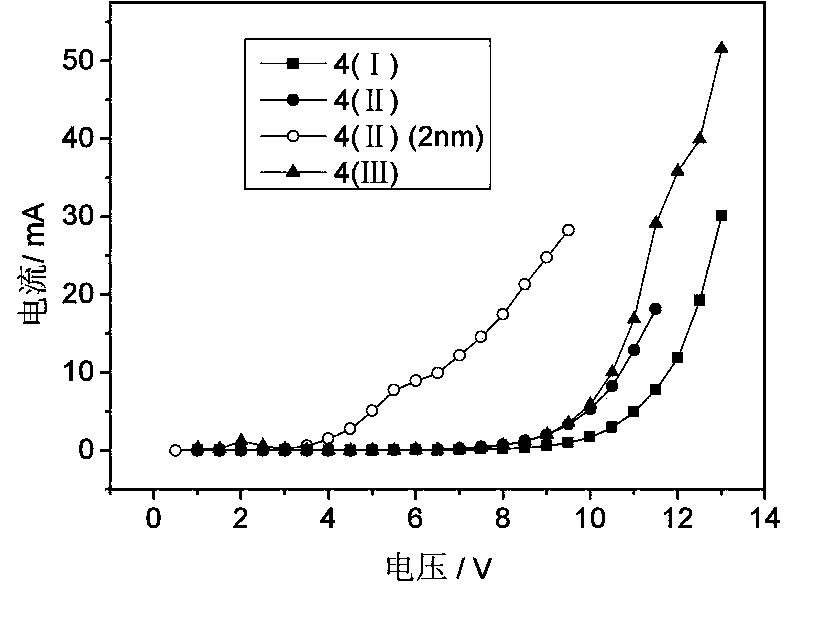
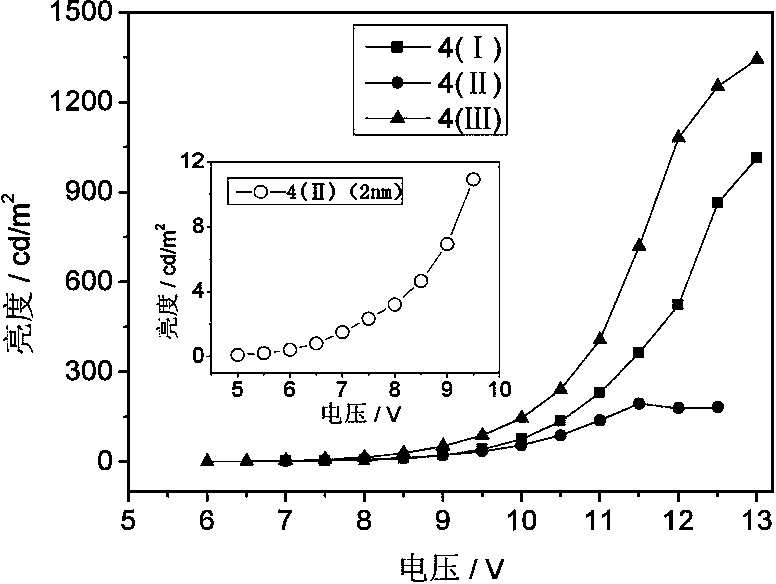
Organic electroluminescent material and application thereof
An electroluminescent material and luminescent technology, applied in luminescent materials, organic chemistry, circuits, etc., can solve the problems of poor voltage stability of red light OLED materials, reduce the interaction of red light materials, and poor voltage and current color stability, etc., to achieve Enhance charge transfer absorption and fluorescence emission intensity, reduce concentration quenching phenomenon, and good voltage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Organic electroluminescence material, structural formula is:
[0032] ...compound 4(I), wherein (2-Ethylhexyl).
[0033] The preparation method of compound 4 (I), comprises the following steps:
[0034]
[0035] Synthesis of compound 6 (2,1,3-benzothiadiazole):
[0036] Add compound 5 (o-phenylenediamine) (10.8g, 0.1mol) and thionyl chloride (53mL) into a three-neck round bottom flask, stir rapidly, and add pyridine (1mL) dropwise into the flask at 0°C , and then the mixture was refluxed for 24h; the excess thionyl chloride was removed by distillation under reduced pressure, and after the system was cooled to room temperature, it was poured into distilled water (250mL); it was purified by steam distillation to obtain a crude product, and then Extracted with methyl chloride (3*50 mL), dried over anhydrous magnesium sulfate, and concentrated the filtrate to obtain a white needle-like compound with a yield of about 82%. Mp=43.7-44.0°C; 1 H-NMR (400MHz, CDCl 3 )...
Embodiment 2
[0048] Organic electroluminescence material, structural formula is:
[0049] ...4(Ⅱ), of which (2-Ethylhexyl).
[0050] The preparation method of compound 4 (II), comprises the following steps:
[0051]
[0052] The synthesis of Compound 6 and Compound 3 refers to Example 1.
[0053] Synthesis of compound 8 (N-ethylhexylphenothiazine):
[0054] Weigh phenothiazine (10g, 31.10mmol) and NaH (1g, 41.70mmol) in a 100ml flask, then add DMF (40ml), stir at room temperature for 30min, add bromoisoctane (8.06g, 41.70mmol), and Stir overnight, add water to the system to stop the reaction, extract three times with trichloro, wash the organic layer with brine, and then wash with anhydrous MgSO 4 Dry overnight, remove the solvent by rotary evaporation, and pass through a column (silica gel, n-hexane:ethyl acetate=9:1, v / v) to obtain a yellow oily liquid. 1 H-NMR (400MHz, CDCl 3 )δ7.15(t, J=7.0Hz, 4H), 6.89(d, J=8.0Hz, 4H), 3.73(s, 2H), 1.93(m, 1H), 1.25-1.44(m, 8H), 0.83-0.93...
Embodiment 3
[0062] Organic electroluminescence material, structural formula is ...4(Ⅲ), of which (2-Ethylhexyl).
[0063] The preparation method of compound 4 (Ⅲ), comprises the following steps:
[0064]
[0065]
[0066] The synthesis of Compound 6 and Compound 3 refers to Example 1.
[0067] Synthesis of compound 9 (4-[(2-ethylhexyl)oxy]-1-iodobenzene):
[0068] Mix 4-iodophenol (4.0g, 18.18mmol), K 2 CO 3 (3.76g, 27.20mmol) and bromoethylhexyl (3.68g, 19.05mmol) in DMF (10mL), heated to 100°C under nitrogen protection, and stirred for 50h. After the reaction, the mixture was poured into 100 mL of distilled water and extracted with dichloromethane (3*50 mL). The organic layer was washed twice with distilled water, dried over anhydrous magnesium sulfate, the solvent was removed under reduced pressure, and purified by column (silica gel, n-hexane) to obtain a colorless liquid compound (3.50 g, 58%). 1 H-NMR (400MHz, CDCl 3 )δ7.51(d, J=8.8Hz, 2H), 6.65(d, J=8.8Hz, 2H), 3.78...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com