Phosphor, Method For Manufacturing Same, Light Emitting Device, And Image Display Device
A technology of phosphors and elements, which is applied in the field of phosphors and can solve problems such as easy decline in the brightness of phosphors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
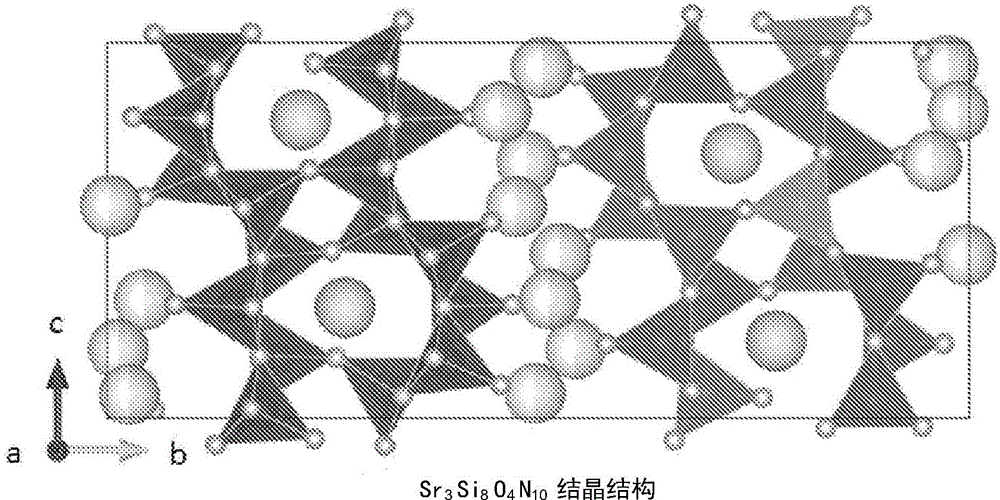
[0208] The preparation method of such a phosphor of the present invention is not particularly limited, for example, by firing a mixture of metal compounds, that is, in an inert atmosphere environment containing nitrogen, within a temperature range of 1200° C. to 2200° C. , to be able to form a Sr 3 Si 8 o 4 N 10 The raw material mixture of inorganic compounds whose crystals are matrix crystals and in which M element is solid-dissolved is burned. The main crystal of the present invention is monoclinic and belongs to space group P2 1 / n, however, depending on the synthesis conditions such as firing temperature, crystals with a different crystal system or space group may be mixed in. In this case, since the change in luminescence characteristics is small, it can be used as a high-brightness fluorescent body.
[0209] As a starting material, for example, a mixture of metal compounds, a compound containing M, a compound containing A, a compound containing D, a compound contain...
Embodiment
[0242] The present invention will be described in more detail by the examples shown below, but they are disclosed only to help understand the present invention more easily, and the present invention is not limited to these examples.
[0243] [Raw materials used in synthesis]
[0244] The raw material powder used in the synthesis has a specific surface area of 11.2m 2 Silicon nitride powder (SN-E10 grade manufactured by Ube Industries, Ltd.), silicon dioxide powder (SiO 2 , High Purity Chemical Research Institute), the specific surface area is 13.2m 2 Alumina powder (Diamicron manufactured by Daemyung Chemical Industry), lithium carbonate (manufactured by High Purity Chemical Industry), boron nitride (manufactured by Denki Chemical Industry), calcium oxide (manufactured by High Purity Chemical Industry), and nitrided with a purity of 99.5% per gram of particle size Strontium (Sr 3 N 2 , manufactured by shellac), strontium oxide (manufactured by High Purity Chemical), ceri...
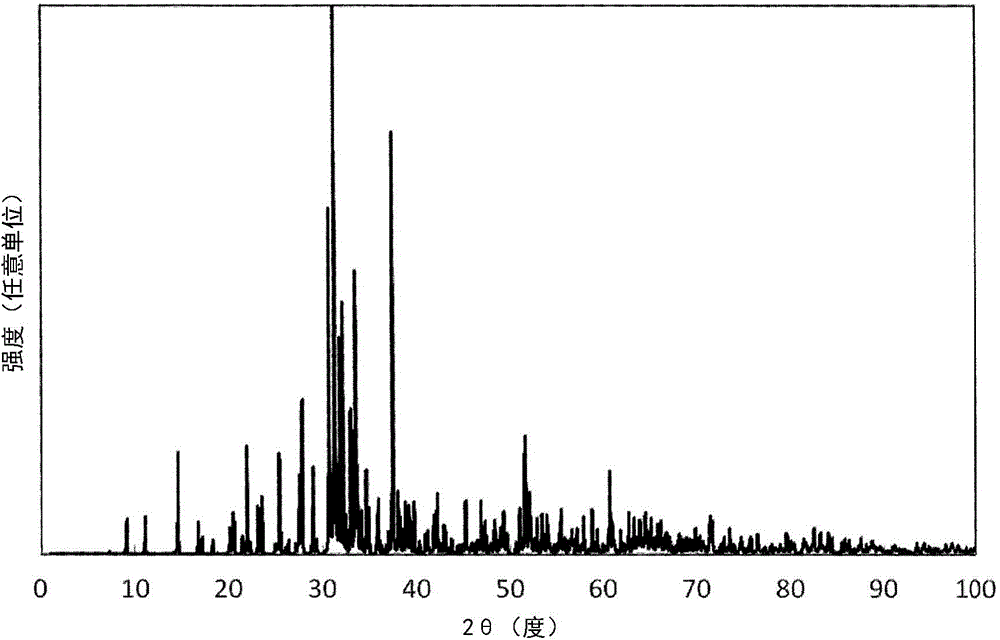
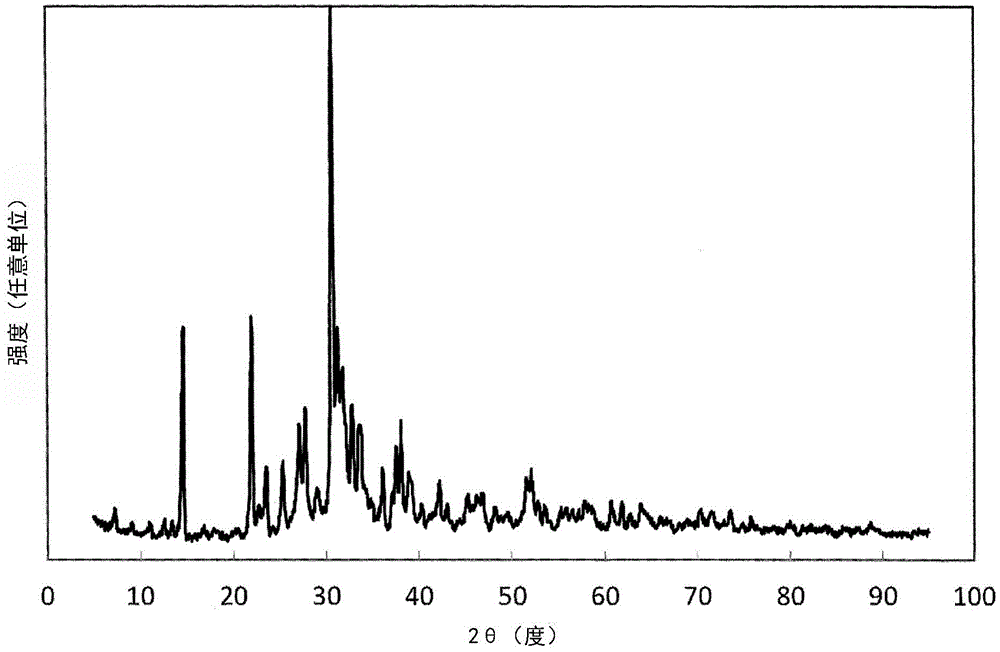
example 1 to example 22
[0255] According to the design composition shown in Table 2 and Table 3, raw materials were weighed so as to obtain the mixing composition (mass ratio) shown in Table 4. The composition differs depending on the type of raw material used, that is, when the design composition in Table 2 and Table 3 is different from the mixed composition in Table 4, the mixed composition is determined so that the amount of metal ions is consistent. The weighed raw material powders were mixed for 5 minutes using a silicon nitride sintered pestle and mortar. Then, the obtained mixed powder was filled in a crucible made of boron nitride. The bulk density of the powder body is about 20% to 30%.
[0256] The crucible filled with the mixed powder was installed on a graphite resistance heating electric furnace. The burning operation is as follows: First, set the burning atmosphere environment to 1×10 through the diffusion pump -1 Vacuum at a pressure below Pa, and heat from room temperature to 800°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com