Separation method suitable for chemical synthesis of salidroside for industrial production
A technology of salidroside and a separation method, applied in the field of salidroside synthesis, can solve the problems of unsuitability for industrial production, cumbersome column separation method, high cost, etc., and achieves reduction of industrial pollution, low production cost, and high production cost. reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
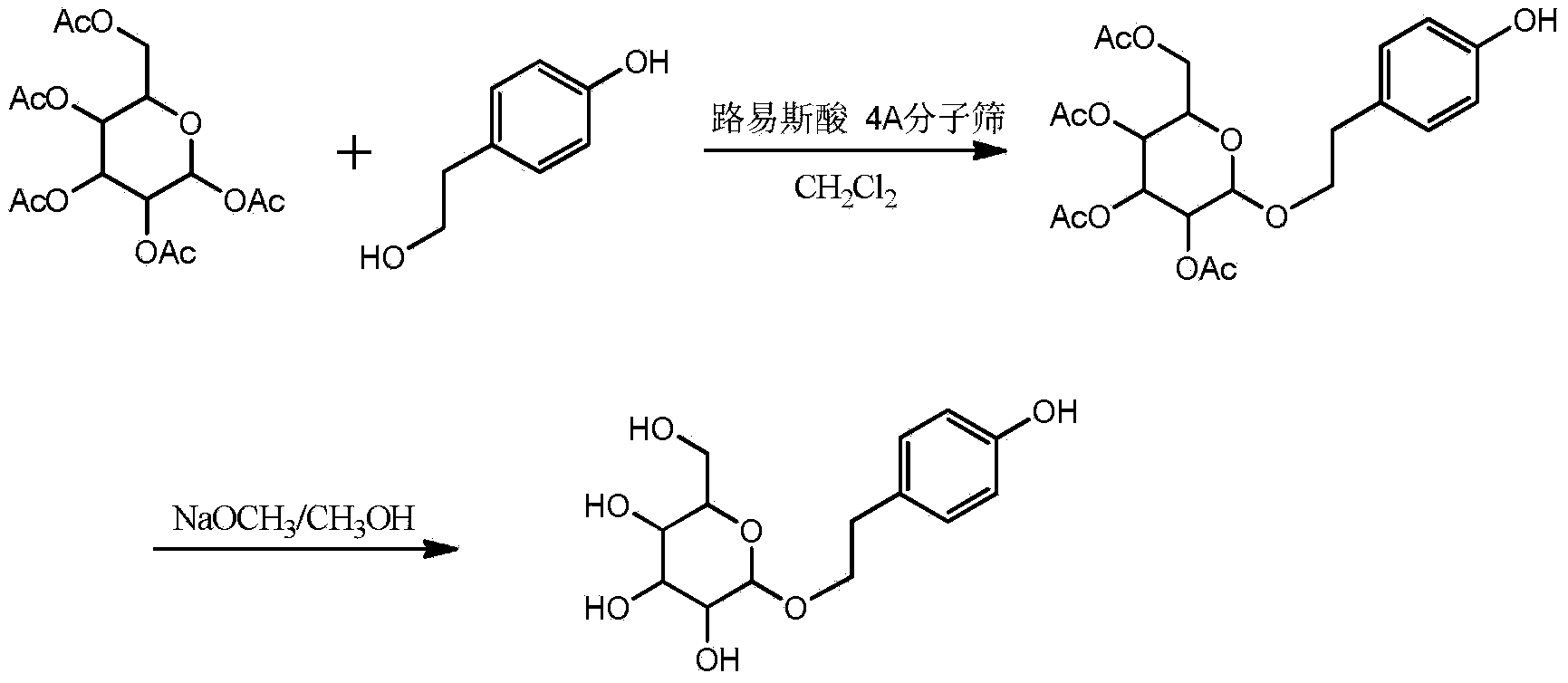
[0025] 1) In a 250ml eggplant-shaped bottle, add a stirring rod, 4g (1.2eq) of β-D-pentaacetylglucose, 1.18g (1eq) of p-hydroxyphenylethyl alcohol and 4g of 4A molecular sieve, and add dry CH 2 Cl 2 60ml was dissolved, and the reactor was placed on a magnetic stirrer, and slowly added dropwise with 10ml of dichloromethane solution containing 2.67g (1.2eq) of anhydrous tin tetrachloride while stirring at 0°C. After the dropwise addition, stirred at 40°C for 2 hour, stop the reaction, now there is a small amount of p-hydroxyphenylethanol remaining, the color of the solution is slightly light yellow, filter out the molecular sieve, and get the reaction solution containing tetraacetylsalidroside, the liquid phase detection tetraacetylsalidroside conversion rate is 94 %.
[0026] 2) In the reaction liquid, add 50ml of 5% sodium bicarbonate aqueous solution while stirring, after stirring, add diatomaceous earth, filter to remove insoluble matter, take the organic phase (that is, th...
Embodiment 2
[0031] 1) In a 250ml eggplant-shaped bottle, add a stirring rod, 4g (1.2eq) of β-D-pentaacetylglucose, 1.18g (1eq) of p-hydroxyphenylethyl alcohol and 4g of 4A molecular sieve, and add dry CH 2 Cl 260ml was dissolved, and the reactor was placed on a magnetic stirrer. Slowly add a 10ml ether solution containing 0.70g (1.2eq) of boron trifluoride dropwise while stirring at 0°C. After the dropwise addition, stir at room temperature for 2 hours to stop the reaction. At this time, there was a small amount of p-hydroxyphenylethanol remaining, and the color of the solution was slightly light yellow, and the molecular sieve was filtered to obtain a reaction solution containing tetraacetylsalidroside. The conversion rate of tetraacetylsalidroside was 92% according to liquid phase detection.
[0032] 2) In the reaction liquid, add 50ml of 5% sodium bicarbonate aqueous solution while stirring, after stirring, add diatomaceous earth, filter to remove insoluble matter, take the organic pha...
Embodiment 3
[0036] 1) In a 250ml eggplant-shaped bottle, add a stirring bar, 4g (1.2eq) of β-D-pentaacetylglucose, 1.66g (1.2eq) of anhydrous ferric chloride, 1.18g (1eq) of p-hydroxyphenylethyl alcohol and 4A Molecular sieve 4g, add dry CH 2 Cl 2 80ml was dissolved, and the reactor was placed on a magnetic stirrer, and stirred at 15°C for 4 hours to stop the reaction. At this time, there was a small amount of p-hydroxyphenethyl alcohol remaining, and the color of the solution was slightly light yellow. Filtered out molecular sieves to obtain tetraacetyl salidroside In the reaction solution, the conversion rate of tetraacetylsalidroside was 92% by liquid phase detection.
[0037] 2) In the reaction liquid, add 50ml of 5% sodium bicarbonate aqueous solution while stirring, after stirring, add diatomaceous earth, filter to remove insoluble matter, take the organic phase (that is, the dichloromethane phase) in the filtrate, use the organic phase Extract with ionic water until the p-hydroxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com