Benzothiazole and triazolediheterocycle-containing fused ring compound and application thereof
A benzothiazole and compound technology, which is applied in the field of bio-organic synthesis, can solve problems such as the inhibition of toxic and side effects of amnafite and mitonatamide, and achieve the effects of improving identification and embedding ability, improving biological activity, and expanding diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
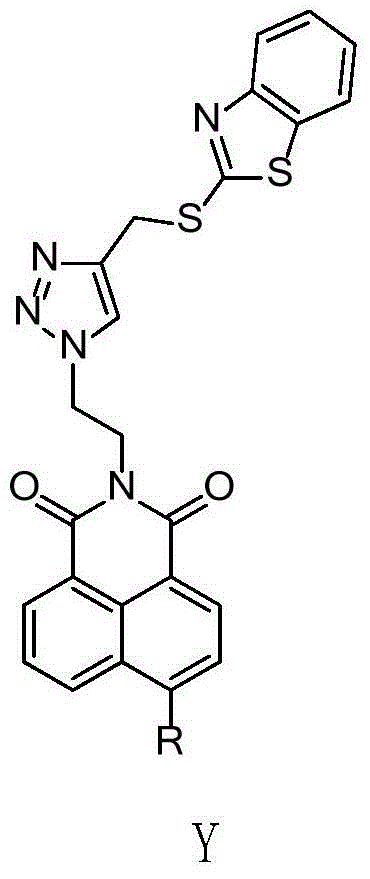
[0050] N-[2'-(4-((Benzothiazole-2-mercapto)methyl)-[1,2,3]-triazole)-ethyl]-4-hexahydropyridine-1,8-naphthalene Synthesis of imide (compound F4):
[0051] (1) Synthesis of 2-(2-alkyne-1-propylthio)benzo[d]thiazole (Intermediate 1)
[0052]
[0053] Weigh 3.02g (18mmol) of 2-mercaptobenzothiazole, dissolve it with 35mL of acetone, add 2.51mL (18mmol) of triethylamine, and the reaction solution is orange transparent liquid, add 1.41mL (18mmol) of propyl bromide alkyne, ultrasonically oscillated for 10 minutes, stopped the reaction, filtered to obtain a brown filtrate, and distilled the filtrate to remove the solvent under reduced pressure. 3.61 g of brown oily solid was obtained, yield: 98.0%.
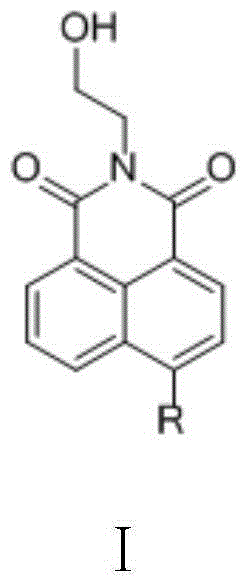
[0054] (2) Synthesis of N-(2'-hydroxyethyl)-4-bromo-1,8-naphthalimide (intermediate 3)
[0055]
[0056] In a 50mL two-necked bottle, add 2.76g (10mmol) 4-bromo-1,8-naphthalene anhydride, add 0.7mL ethanolamine, 28mL absolute ethanol, heat up to reflux under magnetic stirring, r...
Embodiment 2
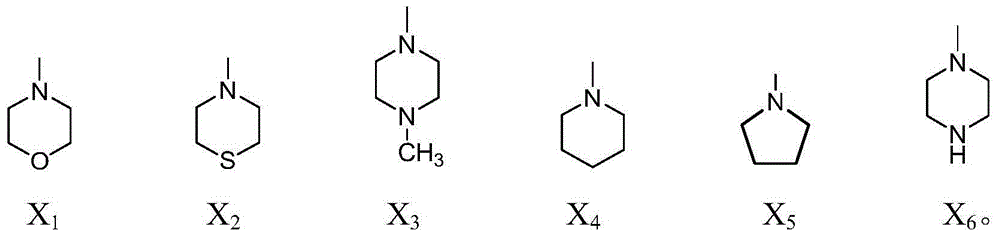
[0072] N-[2'-(4-((Benzothiazole-2-mercapto)methyl)-[1,2,3]-triazole)-ethyl]-4-morpholine-1,8-naphthoyl Synthesis of imine (compound F1):
[0073] Except that morpholine was used to replace hexahydropyridine in (3), other synthesis and experimental treatment methods were the same as in Example 1. Separation by silica gel column chromatography (column chromatography eluent is CH 2 Cl 2 :CH 3 OH=14:1), the target product F1 was obtained as a yellow-green solid with a yield of 79.8%. Melting point: 212.2-215.6°C.
[0074] 1 H NMR (400MHz, CDCl 3 )δ8.48(d, J=7.3Hz, 1H), 8.41(dd, J=13.7, 8.2Hz, 2H), 7.86(d, J=8.0Hz, 1H), 7.74(d, J=8.4Hz, 2H), 7.69–7.62(m, 1H), 7.41(t, J=7.2Hz, 1H), 7.31(d, J=8.0Hz, 1H), 7.18(d, J=8.1Hz, 1H), 4.72( t, J=6.0Hz, 2H), 4.70–4.48(m, 4H), 4.16–3.91(m, 4H), 3.37–3.11(m, 4H). 13 C NMR(101MHz,CDCl3)δ165.99,164.10,163.56,155.97,153.00,135.40,132.85,131.43,130.47,129.92,126.05,126.02,125.79,124.28,123.52,122.58,121.48,121.02,116.28,114.93,66.93,53.40 ...
Embodiment 3
[0077] N-[2'-(4-((Benzothiazole-2-mercapto)methyl)-[1,2,3]-triazole)-ethyl]-4-thiomorpholine-1,8- Synthesis of naphthalimide (compound F2):
[0078] Except replacing hexahydropyridine with thiomorpholine in (3), other synthesis and experimental treatment methods are the same as in Example 1. Separation by silica gel column chromatography (column chromatography eluent is CH 2 Cl 2 :CH 3 OH=6:1), the target product F2 was obtained as a yellow solid with a yield of 75.7%. Melting point: 206.5-208.1°C.
[0079] 1 H NMR (400MHz, CDCl 3)δ8.48(dd, J=7.3,1.0Hz,1H),8.42(d,J=8.1Hz,1H),8.34(dd,J=8.4,1.0Hz,1H),7.85(d,J=7.7 Hz, 1H), 7.75(d, J=7.9Hz, 2H), 7.66(dd, J=8.4, 7.4Hz, 1H), 7.41(t, J=7.2Hz, 1H), 7.30(t, J=7.6 Hz, 1H), 7.19(d, J=8.1Hz, 1H), 4.73(t, J=6.0Hz, 2H), 4.70–4.56(m, 4H), 3.51(s, 4H), 2.99(s, 4H ).
[0080] +ESI MS(M+H):C 28 h 24 N 6 o 2 S 3 , calculated value: 573.1123, measured value: 573.1181.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com