Glucoside containing azobenzene group, and preparation method and application thereof
A technology of azophenyl and nitrogen phenyl, which is applied in the field of catalytic reaction engineering and can solve problems such as difficult to meet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 Synthesis of intermediate 4'-n-butyl-1-azobenzene-2,3,4,6-tetra-O-acetyl-α-D-galactopyranoside
[0100] Specifically include the following steps:
[0101] (1) Coupling reaction of aromatic diazonium salt: Synthesis of compound 4-n-butyl-4'-hydroxyazobenzene (1)
[0102]
[0103] Mix 4-n-butylaniline (0.27mL) with water and concentrated hydrochloric acid and stir evenly for 1h, cool to 0-5°C, add NaNO 2 (116mg) aqueous solution stirring reaction 30min, keep temperature constant, add phenol (158mg) and Na 2 CO 3 (530mg) in aqueous solution, stirring the reaction until the reaction is complete. After the reaction, the pH of the reaction solution was adjusted to ≈3 with concentrated hydrochloric acid. Add 20ml of dichloromethane (CH 2 Cl2 ). use CH 2 Cl 2 Extraction (4×15 mL), combined organic layers, washed with water, washed with saturated NaCl, dried over anhydrous magnesium sulfate. After being dried well, it was filtered, and the filtrate was dist...
Embodiment 2
[0112] Example 2 Synthesis of 4'-n-butyl-1-azobenzene-α-D-galactopyranoside (4)
[0113]
[0114] The orange crystalline product (3) (120mg) obtained in Example 1 was dissolved in anhydrous methanol (2.4mL), triethylamine (0.3mL) and water (0.3mL) were added, and the volume of triethylamine, water and methanol was The ratio was 1:1:8, and the reaction was stirred at room temperature for 24h. After the completion of the reaction as detected by TLC, the solvent was evaporated from the reaction solution under reduced pressure, and the residue was purified by silica gel column chromatography (dichloromethane / methanol) to obtain compound (4) as a pale yellow solid. The yield of compound (4) prepared from compound (2) was 85.8%, and the total yield of compound (4) prepared by the four-step reaction was 58.7%.
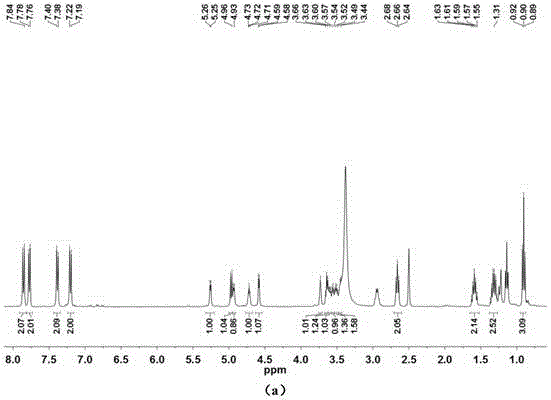
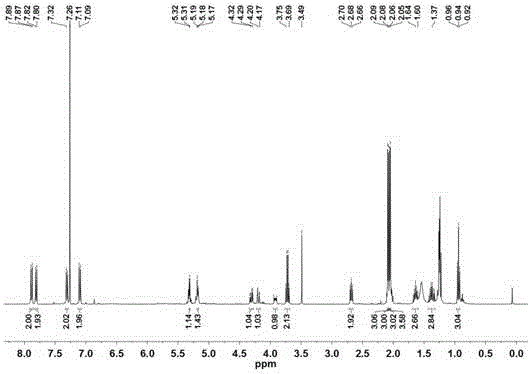
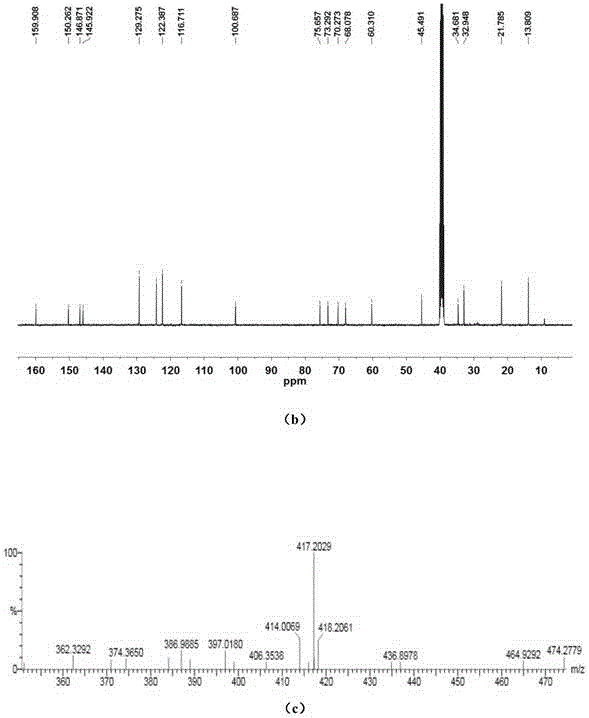
[0115] The compound obtained above is characterized by chemical structure, and its data are as follows:
[0116] 1 HNMR (400MHz, DMSO-d 6 )δ7.86(d, J=8.9Hz, 2H), 7.77(...
Embodiment 3
[0119] Example 3 Synthesis of intermediate 4'-n-heptyl-1-azobenzene-2,3,4,6-tetra-O-acetyl-α-D-galactopyranoside
[0120] Specifically include the following steps:
[0121] (1) Coupling reaction of aromatic diazonium salt: Synthesis of compound 4-n-heptyl-4'-hydroxyazobenzene (5)
[0122]
[0123] Concentrated hydrochloric acid (0.9 mL, 10.8 mmol, 5.4 eq) was added to a mixture of 4-n-heptylaniline (2 mmol, 1 eq) and water (3 mL), and stirred at room temperature for 1 h. The reaction solution was cooled to 0-5°C in an ice-water bath, and the NaNO 2 A solution of (137.99mg, 2mmol, 1eq) in water (2mL) was slowly added dropwise (about 20s / drop) into the reaction flask, and stirred for 30min. Keep 0~5℃, add phenol (188.22mg, 2mmol, 1eq) to the reaction solution, slowly add Na 2 CO 3 (635.94mg, 6mmol, 3eq) in water (7mL) solution, the pH of the reaction solution was measured by pH test paper = 8-10, and the reaction solution was stirred at 0-5°C for 3h. TLC was used to chec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com