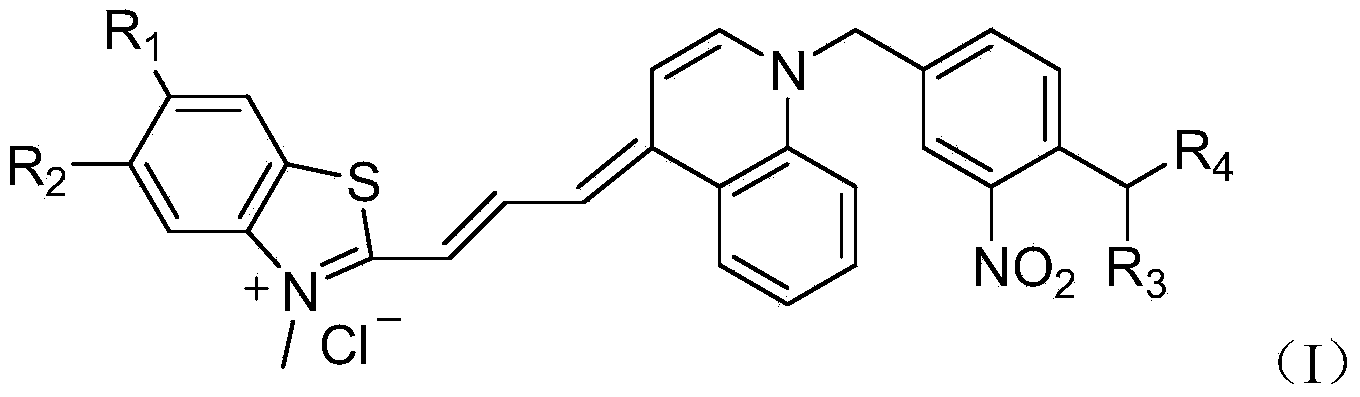
Asymmetric cyanine dye compound and application thereof
A technology of compound and cyanine dye, which is applied in the application field of 650nm channel fluorescence quencher, which can solve the problems of insufficient quenching efficiency and limitation of signal kinetic characteristics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
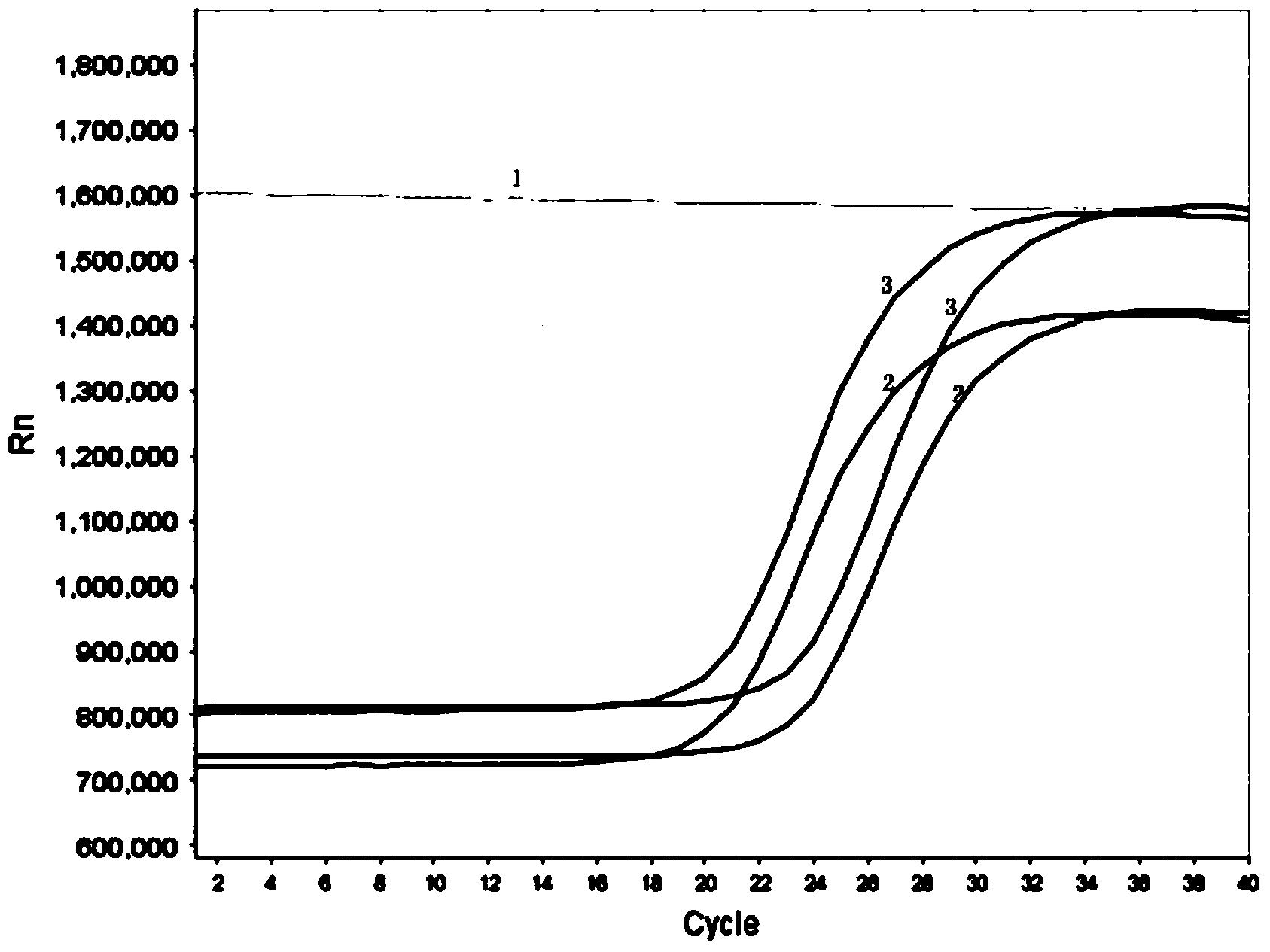
Image
Examples
Embodiment 1
[0033] Embodiment 1: the synthesis of compound (III)
[0034]
[0035] 2,3-Dimethylbenzothiazole p-toluenesulfonate (180mg, 0.52mmol), benzamidine (160mg, 0.8mmol) and 2ml of acetic anhydride were refluxed for 30 minutes, and after cooling, they were dropped into diethyl ether to obtain 170mg of the product .
[0036] Product NMR data 1 H NMR: 1.82 (s, 3H), 2.34 (s, 3H), 6.06 (m, 1H), 7.6 (m, 1H), 4.39 (s, 3H), 7.5-8.6 (m, 13H).
Embodiment 2
[0037] Embodiment 2: the synthesis of compound (II)
[0038]
[0039] 4-Methylquinoline (4.8ml, 0.0363mol) with methyl 2-(4-(bromomethyl)-2-nitrophenyl)propionate (according to Naoki Yamakawa et al., Bioorganic & Medicinal Chemistry 19(2011) 3299 -3311 preparation) (16.44g, 0.05445mol) were mixed, reacted overnight at 110°C, after cooling, added 20ml of methanol to dissolve, suspended and steamed to obtain a viscous solid, then added 200ml of acetone and stirred, after stirring for 2h, the product precipitated, filtered , dried to obtain product 6.4g.
[0040] Product NMR data 1 H NMR: 1.51 (s, 3H), 2.66 (s, 3H), 3.6 (d, 3H), 3.7 (m, 1H), 5.95 (s, 2H), 7.5-8.6 (m, 9H).
Embodiment 3
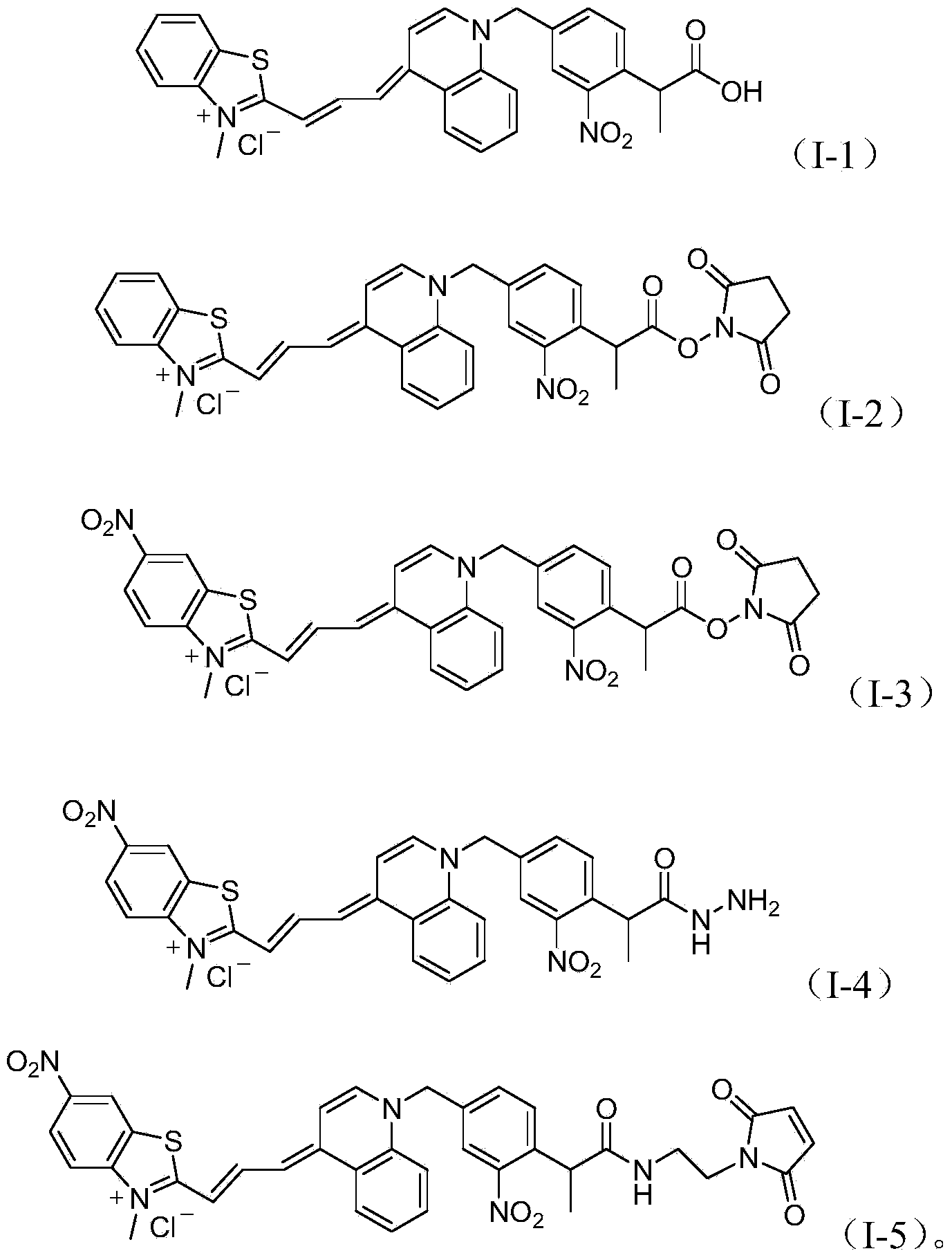
[0041] Embodiment 3: the synthesis of compound (I-1)
[0042]
[0043] Compound III (60mg0.13mmol) and compound (II) (89mg, 0.2mol) were dissolved in 2ml of pyridine, heated to reflux for 30 minutes, after the reaction was completed, the pyridine was removed by rotary evaporation, the residue was poured into water, and 5% hydrochloric acid was added dropwise , filter after the product is precipitated, dissolve the product obtained by filtration in 30ml of 1:1 methanol and water, add 80mg of sodium hydroxide for hydrolysis, after hydrolysis, drop concentrated hydrochloric acid again, after the product precipitates, filter and dry to obtain the final product.
[0044] Product NMR data 1 H NMR: 1.56(m, 3H), 3.82(m, 1H), 4.39(s, 3H), 5.5(m, 3H), 6.65(m, 1H), 6.71(m, 1H), 8.54(m, 1H ), 7.3-8.6(m, 12H),
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com