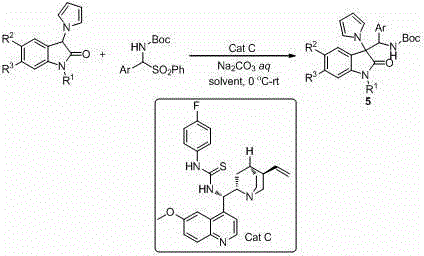
3, 3'-disubstituted 3-pyrrole oxoindole compound and asymmetric synthetic method thereof
A technology of indole compound and synthesis method, which is applied in the field of 3-pyrrole oxindole compound and its asymmetric synthesis, achieving the effects of mild reaction conditions, simple operation and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
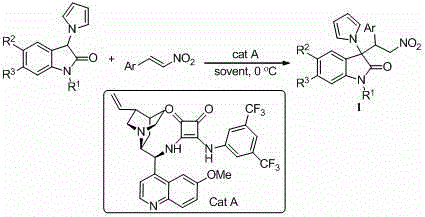
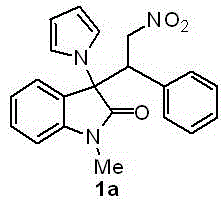
[0037] Embodiment 1 The compound shown in the following structural formula 1a and asymmetric synthesis
[0038]
[0039] Synthetic method: In a hard reaction tube, 1-methyl-3-(1 H -Pyrrol-1-yl)indolin-2-one (21.2 mg, 0.1 mmol), catalyst 3-((3,5-bis(trifluoromethyl)phenyl)amino)-4-((( S)-(6-methoxyquinuclidin-4-yl)((1S,2S,4S,5R)-5-vinylquinuclidin-2-yl)methyl)amino)cyclobut-3-ene -1,2-dione (3.2 mg, 0.005 mmol) and ( E )-(2-nitrovinyl)benzene (22.4 mg, 0.15 mmol) was dissolved in 2 mL of freshly distilled dichloromethane, and the mixture was o The reaction was stirred at C for 54 h. After the reaction was monitored by TLC, the mixture was directly separated and purified by column chromatography (the mobile phase was petroleum ether:ethyl acetate=10:1~3:1) to obtain a white solid with a yield of 97%. After structural identification, the structure of the solid was For the above-mentioned compound 1a, the specific data of its structural identification are:
[0040] The...
Embodiment 2
[0041] Embodiment 2: the compound of structural formula as shown below 2a and asymmetric synthesis
[0042]
[0043] Synthetic method: In a hard reaction tube, 1-methyl-3-(1 H -pyrrol-1-yl)indolin-2-one (21.2 mg, 0.1 mmol), (DHQD) 2 PHAL (7.8 mg, 0.01 mmol) and diethyl azodicarboxylate (26.1 mg, 0.15 mmol) were dissolved in 2 mL of freshly distilled chlorobenzene, and the mixture was stirred at -20°C for 60 h. After the reaction was monitored by TLC, the mixture was directly separated and purified by column chromatography (the mobile phase was petroleum ether: ethyl acetate=10:1~2:1) to obtain a white solid with a yield of 98%; through structural identification, the structure of the solid For the above-mentioned compound 2a, the specific data of its structural identification are:
[0044] The ee value is 80%. [α] D 20 = +113.6 ( c 1.57, CHCl 3 ); mp 181.3-182.7 oC; the ee was determined by HPLC (Chiralpak OD-H, EtOH / hexane = 5 / 95, flow rate 1.0 mL / min, lambda = ...
Embodiment 3
[0045] Embodiment 3: the compound of structural formula as shown below 3a and asymmetric synthesis
[0046]
[0047] Synthetic method: In a hard reaction tube, 1-methyl-3-(1 H -Pyrrol-1-yl)indolin-2-one (21.2 mg, 0.1 mmol), catalyst 1-(3,5-bis(trifluoromethyl)phenyl)-3-((1S,2S) -2-(Dimethylamino)cyclohexyl)thiourea (8.3 mg, 0.02 mmol) and 1-(p-methylphenyl)-1 H -Pyrrole-2,5-dione (28.1 mg, 0.15 mmol) was dissolved in 2 mL of freshly distilled dichloroethane, and the mixture was stirred at room temperature for 6 days. After the reaction was monitored by TLC, the mixture was directly separated and purified by column chromatography (the mobile phase was petroleum ether:ethyl acetate=10:1~2:1) to obtain a white solid with a yield of 56%; after structural identification, the structure of the solid For the above-mentioned compound 1a, the specific data of its structural identification are:
[0048] The dr value is 85:15, and the ee value is 93%. [α] D 20 = -232.4 ( c 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com