A pharmaceutical composition comprising imipenem cilastatin sodium and its preparation
A technology of cilastatin sodium and imipenem, which can be used in medical preparations containing active ingredients, medical preparations without active ingredients, antibacterial drugs, etc., can solve problems such as large adverse drug reactions, and reduce the The incidence of central adverse reactions, the improvement of stability, and the effect of reducing central adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
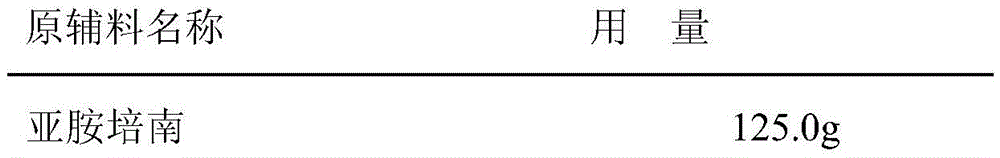
[0024] Embodiment 1 powder injection of the present invention
[0025] Prescription composition:
[0026]
[0027] Preparation Process:
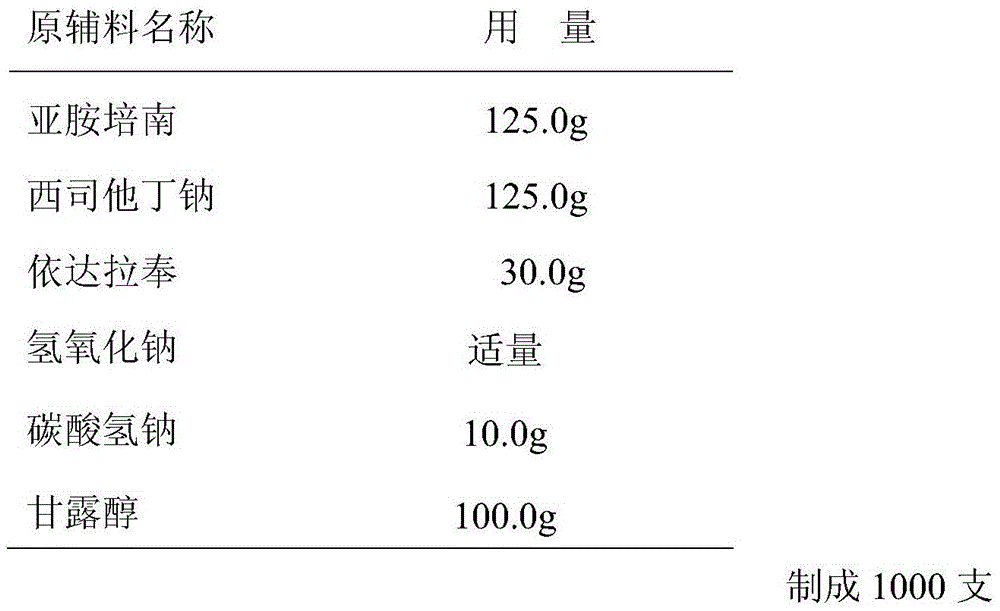
[0028] Weigh the prescribed amount of imipenem, cilastatin sodium, edaravone and auxiliary materials; add sodium bicarbonate and mannitol into an appropriate amount of water for injection, stir and dissolve, add the prescribed amount of imipenem, cilastatin For statin sodium and edaravone, heat and boil to dissolve the drug completely and use sodium hydroxide to adjust the pH value of the drug solution to 7.0-8.5. Add 0.1% activated carbon to the drug solution and stir for 15 minutes. 0.45 μm and 0.22 μm filter to remove activated carbon, after the filtrate was cooled to room temperature, add 6 times the volume of absolute ethanol to the drug solution, a precipitate formed in the solution, after stirring for 30 minutes, use a 0.45 μm filter to obtain an off-white solid. The off-white solid is washed with absolute ethanol, vacuum-dried, ...
Embodiment 2
[0029] Embodiment 2 powder injection of the present invention
[0030] Prescription composition:
[0031]
[0032] The preparation method is the same as Example 1 of the present invention except that the prescription is different.
Embodiment 3
[0033] Embodiment 3 powder injection of the present invention
[0034] Prescription composition:
[0035]
[0036] The preparation method is the same as Example 1 of the present invention except that the prescription is different.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com