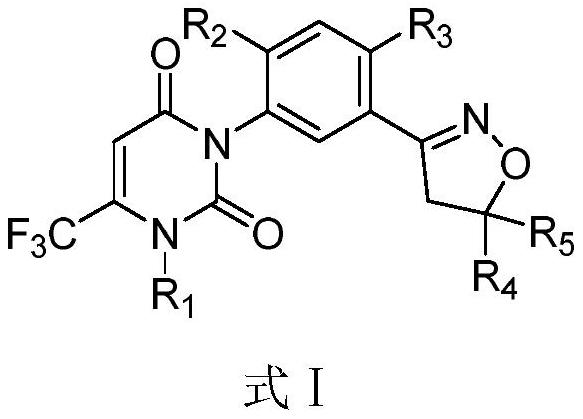
Patents
Literature
40results about How to "The content of active ingredients is stable" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of cistanche deserticola phenylethanoid glycosides
InactiveCN102441040AAvoid decompositionPrevent oxidationNervous disorderDigestive systemStability studyGlycoside formation
The invention relates to a preparation method of cistanche deserticola phenylethanoid glycosides. The method comprises the steps of: cistanche deserticola crushing, lixiviating, absorbent resin adsorption, elution, and concentration, etc. In research of the extraction and stability of cistanche deserticola phenylethanoid glycoside, under a weakly acidic condition with pH of 2-4, the cistanche deserticola phenylethanoid glycoside can have substantially improved stability, and under the condition, the cistanche deserticola phenylethanoid glycoside extracted from cistanche deserticola is mainly composed of echinacoside and verbascoside. And in cistanche deserticola phenylethanoid glycosides, the total content proportion of echinacoside and verbascoside is greater than 90%. The content of cistanche deserticola phenylethanoid glycosides extracted by the method of the invention is obviously higher when compared with other extraction methods. And the obtained cistanche deserticola extract has stable effective component content and relative light color. The method provided in the invention avoids decomposition and oxidation of cistanche deserticola phenylethanoid glycoside, and is in favor of product stability.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Compound traditional Chinese medicine preparation for treating tumors and preparation method thereof
ActiveCN102008650ASimple and fast operationShort cycleAntineoplastic agentsPlant ingredientsMedicinePolyvinyl alcohol
The invention provides a compound traditional Chinese medicine preparation for treating tumors and a preparation method thereof. The compound traditional Chinese medicine preparation is characterized by being prepared from the following raw materials in parts by weight: 1-4 parts of pinellia ternate, 1-4 parts of selfheal, 0.75-3.5 parts of sagittaria sagittifolia L and 0.75-5.5 parts of varech. The preparation method is characterized by smashing the raw materials into coarse powder; utilizing water or polyvinyl alcohol aqueous solution containing ethanol to extract effective ingredients from the coarse powder; and according to the specification requirements of oral preparation, extracting, concentrating and pelletizing to obtain the oral preparation. The compound traditional Chinese medicine preparation for effectively treating tumors and the preparation method of the preparation, which are provided by the invention, are verified by research on cell experiments and animal entity tumor experiments of the compound traditional Chinese medicine preparation; and better curative effect is obtained through clinical indication medications.
Owner:天津市医药科学研究所
Method for preparing nalmefene hydrochloride injection and prepared nalmefene hydrochloride injection
InactiveCN103202806AExcellent pHImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSample appearanceAdditive ingredient
The invention discloses a method for preparing nalmefene hydrochloride injection. The method comprises the steps of adding a certain amount of citric acid or citrate auxiliary material to the preparation when the nalmefene hydrochloride injection is prepared according to a common method, wherein the adding amount is 5-15g of citric acid or citrate added to each liter of injection; firstly, taking the prescribed auxiliary material in preparation, adding injection water to dissolve, adding active carbon to agitate and adsorb, filtering and removing impurities; adding the prescribed nalmefene hydrochloride under the protection of nitrogen, fully dissolving and sizing to an appointed constant volume by water, adjusting the pH value to 3.5-4.5; and finally conducting split charging, charging nitrogen, sealing and sterilizing after filtering by a filter. By adopting the method disclosed by the invention, the validity period of the nalmefene hydrochloride injection can be obviously prolonged; a specific citric acid pH value stabilizer is adopted; and the nalmefene hydrochloride injection has good stability, and stable sample appearance, pH value, effective ingredient content and related substances, and the like. Therefore, the safety of medication is ensured.
Owner:ANHUI HEALSTAR PHARM CO LTD
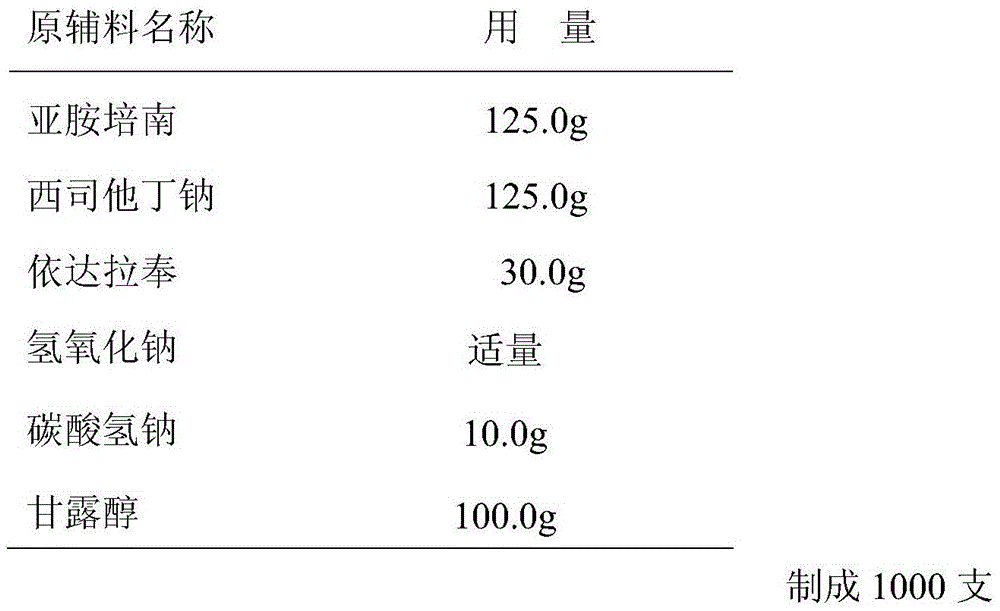
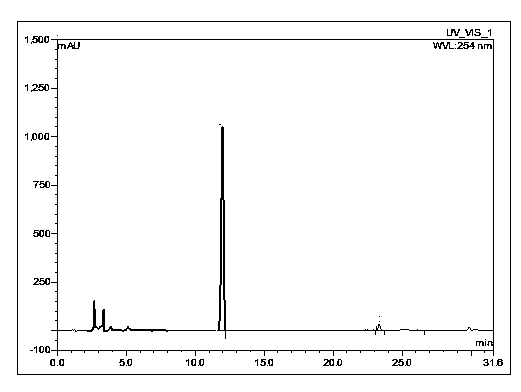
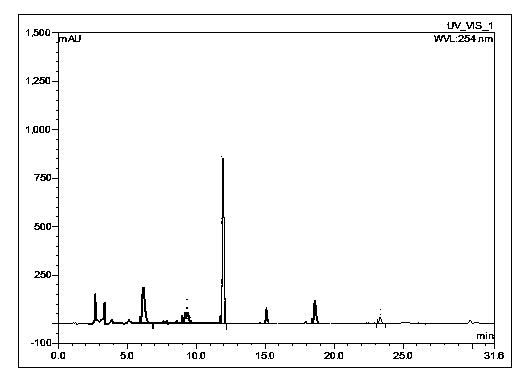
Pharmaceutical composition containing imipenem cilastatin sodium and preparation thereof
ActiveCN104095847AHigh antibacterial activityImprove stabilityAntibacterial agentsOrganic active ingredientsCilastatin sodiumAdverse effect
The invention relates to a pharmaceutical composition containing imipenem cilastatin sodium and preparation thereof, belongs to the field of medicine and aims at overcoming the technical shortcoming that an imipenem cilastatin sodium powder injection in the prior art causes large adverse effect on the centre. The invention provides a powder injection containing the imipenem cilastatin sodium through reasonable compatibility. The powder injection can effectively reduce the adverse effect on the centre of an imipenem cilastatin sodium compound preparation when being used for clinic antibacterial treatment, the stability of the re-dissolved powder injection can be remarkably improved, and therefore, the pharmaceutical composition is suitable for development of clinic treatment medicine.
Owner:CHINA NAT MEDICINES GUORUI PHARMA
Method for preparing 'Sanjin tablet'
ActiveCN1628814AShorten drying timeLight colorUnknown materialsUrinary disorderSmilax chinaLygodium japonicum
The invention discloses a method for preparing 'Sanjin tablet' which comprises, (1) extracting Fructus Rosae Laevigatae, sheep opening, smilax China root, Lygodium japonicum, cat's-foot with water, (2) mixing the extract with pharmaceutically acceptable carrier, spray drying and granulating, (3) tabletting, (4) dressing.
Owner:GUILIN SANJIN PHARMACEUTICALS CO LTD
Preparation technology of radix salviae miltiorrhizae tablets
InactiveCN110496152AReduce subsequent degradationImprove stabilityPharmaceutical non-active ingredientsDrageesAlcoholSalvianolic acid B
The invention provides a preparation technology of radix salviae miltiorrhizae tablets. The preparation technology comprises the steps of cutting radix salviae miltiorrhizae medicinal materials in different batches into segments, detecting the extracts of radix salviae miltiorrhizae decoction pieces, and detecting the content of effective components, wherein (Chinese Pharmacopoeia) the radix salviae miltiorrhizae medicinal materials contain 3.0% or above of Salvianolic acid B, the total extracts are not less than 50.0%, according to different extracts and the content of the effective components, preparation is performed, so that the total extracts are 65%-75%, and the content of the Salvianolic acid B is 7%-9%; placing the decoction pieces prepared in the proportion in an extracting tank,adding 90% alcohol, performing extraction for 1.0 hour, and concentrating outlet liquid; adding water, and performing extraction for 1.5 hours; concentrating outlet liquid; merging the concentrated liquid, and then performing concentration once again; and performing spray drying, collecting powder, then performing granulation, and performing tabletting so as to obtain the radix salviae miltiorrhizae tablets. The preparation technology is suitable for industrial production.
Owner:上海蔡同德堂中药制药厂有限公司
Honeysuckle throat clearing tablets and preparation method thereof
ActiveCN102657778AGood disintegration timeImprove appearancePharmaceutical non-active ingredientsPill deliveryThroatHoneysuckle
The invention belongs to the field of medicinal preparations, and particularly relates to a honeysuckle throat clearing formula and a tablet preparation method thereof. The honeysuckle throat clearing tablets contain medicament extract and pharmaceutically acceptable diluting agent, lubricating agent and disintegrating agent. The honeysuckle throat clearing tablets prepared by the invention have a better treatment effect on acute pharyngitis.
Owner:GENERAL HOSPITAL OF PLA
Tripterygium wilfordii regenerated plant and its preparation process
InactiveCN1366810AHigh reproductive coefficientStable contentHorticulture methodsPlant tissue cultureAlcohol contentMass propagation
The preparation method of regenerative plant of thunder god vine mainly utilizes the techniques of clonal variation, mutation, chromosome doubling, regeneration and hormoneless propagation, etc. Saidinvention not only can make mass propagation, and the propagation coefficient of every generation can be up to 5-6 times, and the triptolide alcohol content is 0.009-0.015%, and its total diterpene lactone content also is higher than that of commercial thunder god vine extract, and they can be retained stably.
Owner:上海延农生物工程有限公司 +2
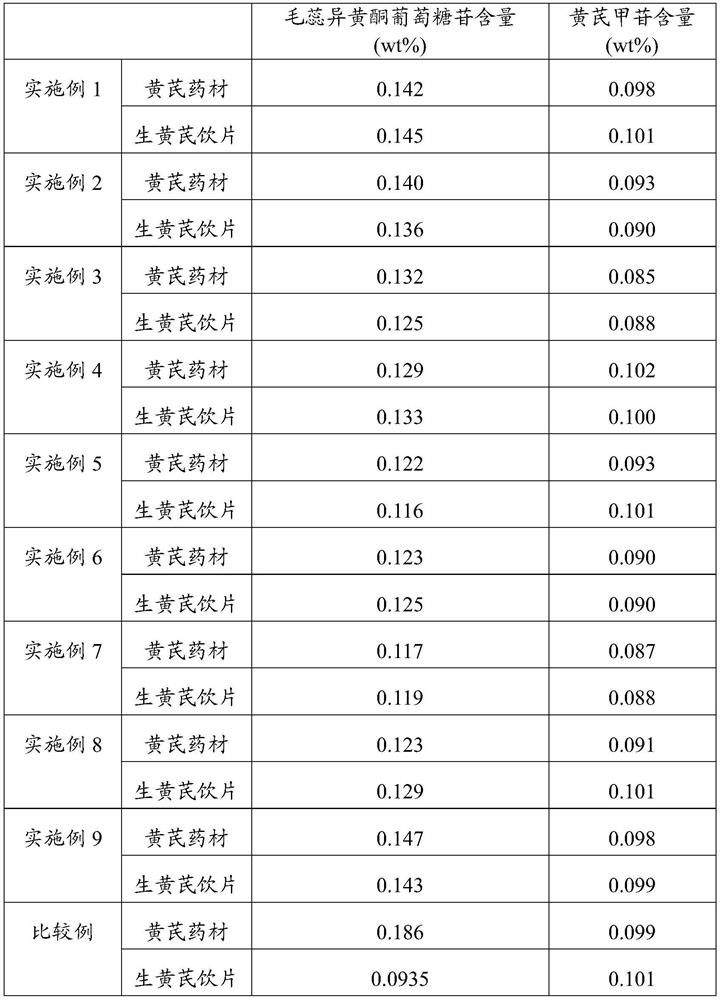
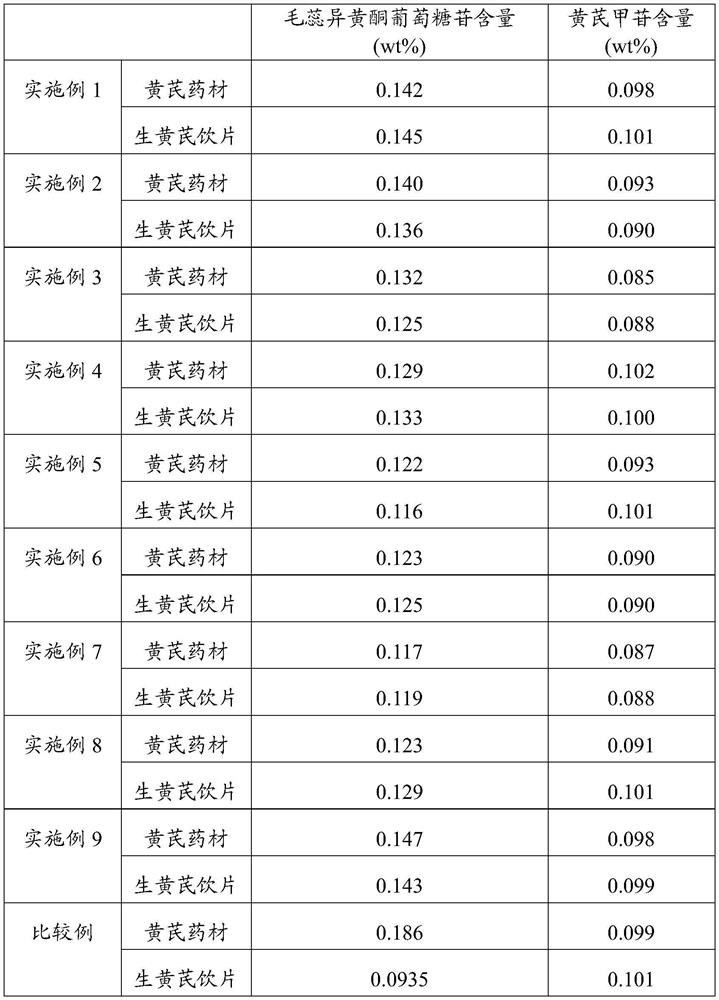
Processing method of raw radix astragali decoction pieces and raw radix astragali decoction pieces
ActiveCN113713006AReduce contentContent unchangedAntibacterial agentsAntimycoticsAstragalosideRadix Astragali seu Hedysari
The invention discloses a processing method of raw radix astragali decoction pieces and the raw radix astragali decoction pieces. The processing method comprises the following steps that (1), heat treatment is carried out on cleaned and selected radix astragali to obtain radix astragali subjected to heat treatment; and (2), the radix astragali subjected to heat treatment is sequentially moistened, sliced and dried to obtain the raw radix astragali decoction pieces. By means of the processing method, the content of calycosin-7-glucoside in the raw radix astragali decoction pieces can be increased, and the high astragaloside content can be kept.
Owner:江西景德中药股份有限公司
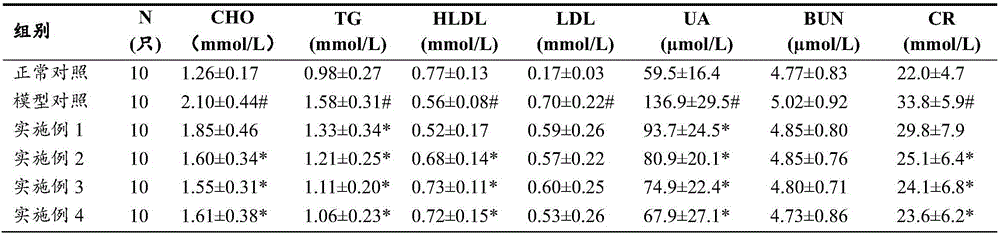
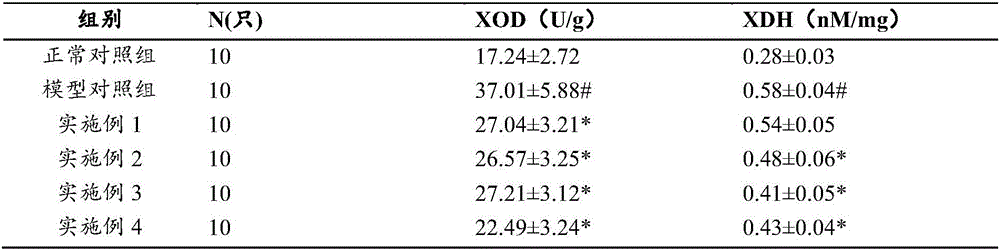
Chinese medicine composition for preventing and treating hyperuricemia and hyperlipemia and preparation method thereof
ActiveCN105749072AMeet the characteristics of long-term medicationWith dehumidification and seepageMetabolism disorderSkeletal disorderCannabisSide effect
The invention discloses a Chinese medicine composition for preventing and treating hyperuricemia and hyperlipemia and a preparation method thereof, and relates to medicine or health-care food for preventing and treating hyperuricemia and hyperlipemia.The Chinese medicine composition for preventing and treating hyperuricemia and hyperlipemia contains extract of a Chinese medicine formula, and according to the formula, the Chinese medicine composition is prepared from, by weight, 3-20 parts of coix seeds, 5-40 parts of glabrous greenbrier rhizome, 3-20 parts of plantain herbs, 1-9 parts of Chinese atractylode rhizome, 1-12 parts of the root of bidentate achyranthes, 1-9 parts of papaw and 1-9 parts of fructus cannabis.The Chinese medicine composition has the functions of clearing away dampness, eliminating water seepage and inducing diuresis for treating stranguria, can obviously regulate and treat the hyperuricemia and hyperlipemia and is free of toxic and side effects after being taken for a long time; the preparation technology is stable, and production feasibility is good.The medicine or health-care food for preventing and treating the hyperuricemia and hyperlipemia contains an effective dose of the Chinese medicine composition for preventing and treating the hyperuricemia and hyperlipemia.
Owner:SHENZHEN ELDERLY MEDICAL RES INST +2
Herbicide composition dispersible oil suspending agent and preparation method thereof
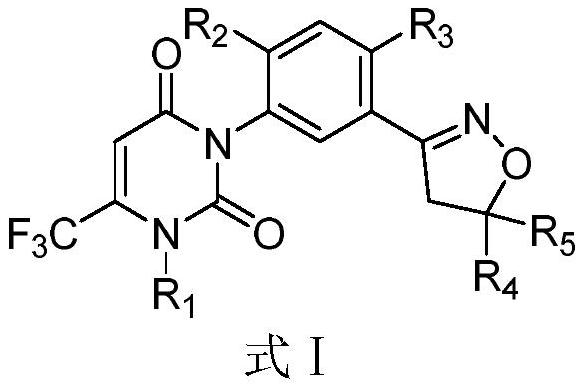
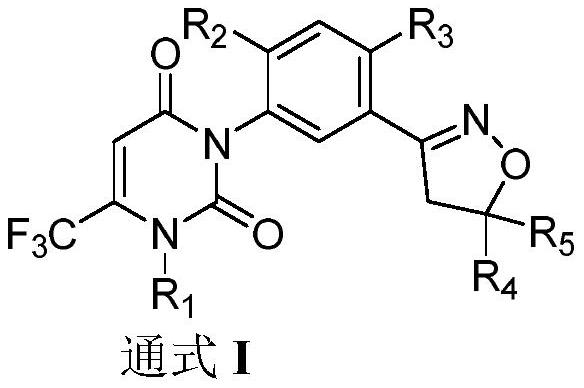
ActiveCN113491269AGood dispersion effectOvercoming easily decomposedBiocideAnimal repellantsWeedZinnia elegans
The invention belongs to the field of agricultural herbicides, and relates to a herbicide composition dispersible oil suspending agent and a preparation method thereof. The invention also relates to the use of the dispersible oil suspending agent for preventing unwanted plant growth. The dispersible oil suspending agent comprises the following components in percentage by weight: 0.1-20% of an active component A, 1-60% of an active component B, 1-30% of a surfactant, 0.1-5% of a pH regulator, 0.5-5% of a structure stabilizer, 0-20% of a solvent and the balance of an oil base, wherein the active component A is a compound as shown in a formula I, and the active component B is selected from glyphosate and agrochemically applicable salts or esters thereof. The dispersible oil suspending agent provided by the invention is stable and free of physical unstable phenomena such as creaming and bottom formation during heat storage, and the effective components are not easy to decompose. The herbicide composition disclosed by the invention can be used for preventing and treating various weeds, especially broadleaf weeds such as piemarker and zinnia elegans, and has an excellent prevention effect.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD +1
Preparation method of herba centellae substitutional tea
The invention discloses a preparation method of herba centellae substitutional tea. The preparation method comprises the steps of (1) collecting: collecting centella leaves in two seasons of summer and autumn; (2) cleaning and selecting: removing foreign matters, branches or yellow leaves; (3) spreading for cooling: ventilating and spreading for cooling the centella at 26 to 30 DEG C; (4) de-enzyme: performing de-enzyme treatment on the centella leaves; (5) molding: putting raw materials after de-enzyme on rolling equipment, spreading for cooling firstly, then rolling to obtain strip decoction pieces; (6) drying: drying the rolled leaves under the condition that the temperature is 50 to 110 DEG C and the thickness is 1.0 to 3.0 cm, and the drying time is 1 to 2h; (7) controlling the humidity of the dried herba centellae substitutional tea to below 60 percent, and cooling the dried herba centellae substitutional tea to room temperature, and then performing subpackage to obtain a finished product. According to the preparation method of the herba centellae substitutional tea, all aspects such as the purity of materials, sensation of sense organs of the color, shape, smell and the like of the tea, the mobility of yield production, moisture and ash content, the content of active ingredients, long-term stability, brewing property of the decoction pieces, and the like are obviously superior to that of the existing technical scheme.
Owner:GUANGZHOU HANFANG PHARMA
A kind of pharmaceutical composition containing zoledronic acid and its preparation
ActiveCN104095863BGood synergyLower ratioPowder deliveryOrganic active ingredientsBone densityWeight coefficient
The invention relates to a pharmaceutical composition containing zoledronic acid and a preparation thereof, belongs to the field of medicine and aims at overcoming the technical shortcoming that osteoporosis therapeutic drug is poor in symptom control effect after malignant tumor radiation and chemotherapy in the prior art and providing a pharmaceutical composition containing the zoledronic acid. The pharmaceutical composition is prepared into a freeze-dried powder injection, has good symptom control effect when being used for osteoporosis therapy after malignant tumor radiation and chemotherapy, has remarkable therapeutic effect on the aspects of bone mineral density improvement, bone weight coefficient increase and blood phosphor and calcium content rise and is suitable for treatment of osteoporosis caused by tumor chemotherapy.
Owner:CHINA NAT MEDICINES GUORUI PHARMA
Processing method of raw astragalus decoction pieces and raw astragalus decoction pieces
ActiveCN113713006BReduce contentContent unchangedAntibacterial agentsAntimycoticsTraditional medicineIsoflavones
Owner:江西景德中药股份有限公司
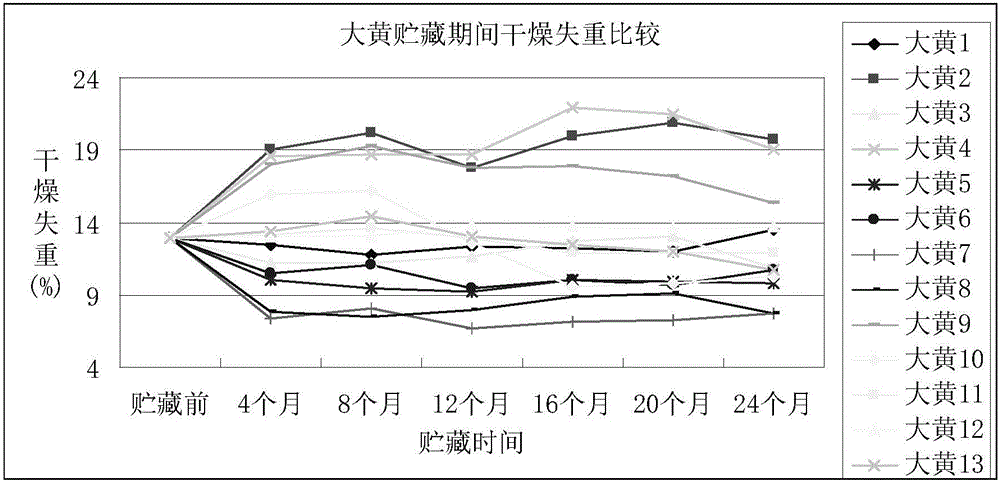
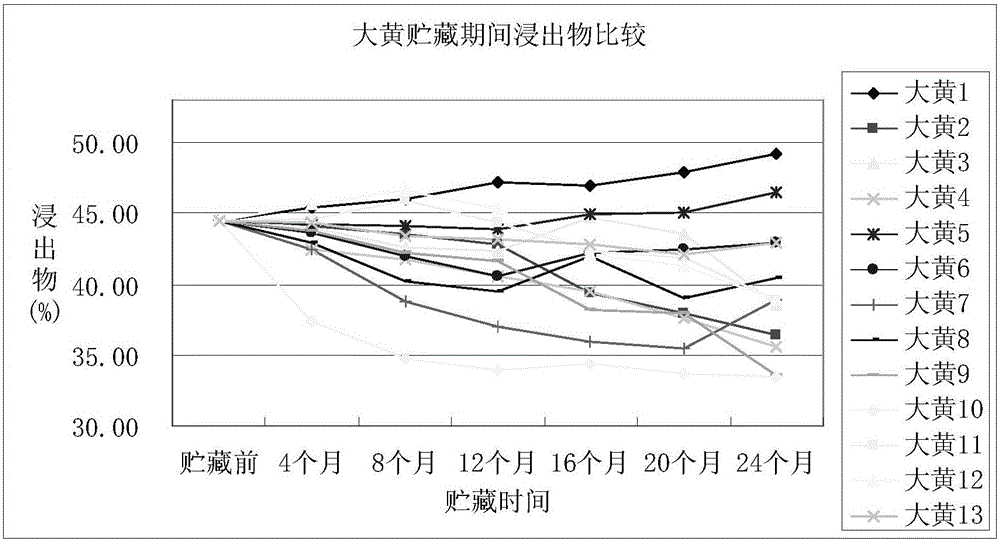
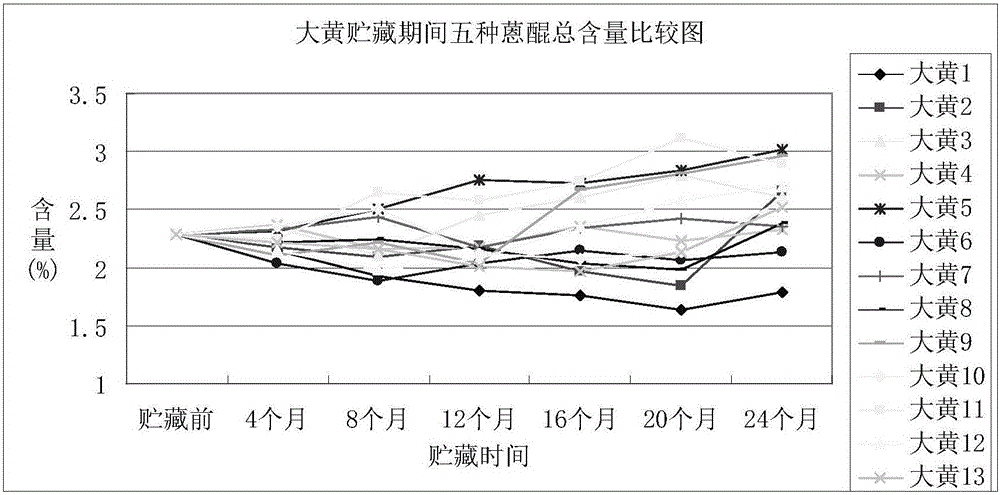
Storage method of rhubarb
InactiveCN105902628AThe content of active ingredients is stableGuaranteed qualityPlant ingredientsMedicineAntagonist
The invention discloses a storage method of rhubarb. The method comprises the following steps of: (1) pretreatment, namely treating rhubarb by <60>Co radiation or intermittent freezing; and (2) storage, namely packaging the rhubarb treated in step (1) with woven bags or plastic bags, and storing the rhubarb using quick lime or glycerine as an antagonist at normal temperature or low temperature in a sealed and shaded mode. The storage method of rhubarb can maintain the quality and medicinal effect of the rhubarb effectively for long time, and has good application prospect.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
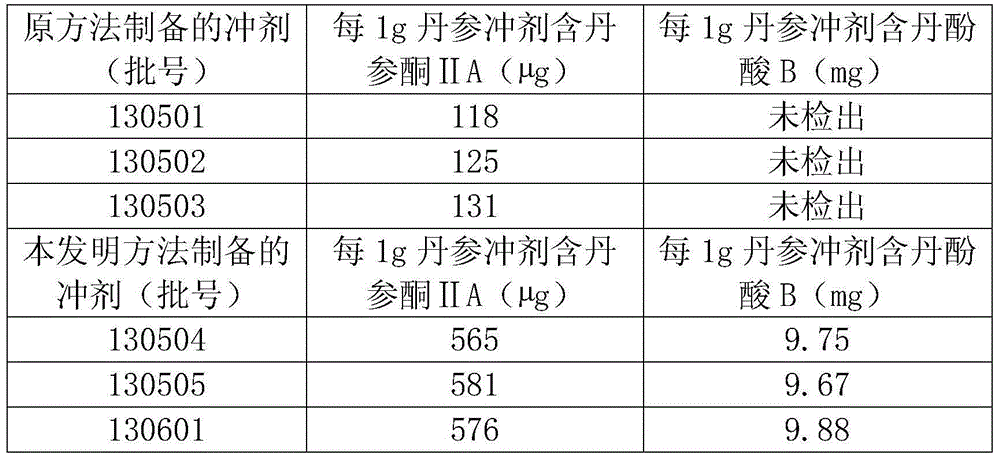
Preparation method and detection method of salvia miltiorrhizagranules
InactiveCN104474016AAvoid lostReduce heat lossComponent separationGranular deliveryBiotechnologyMedicinal herbs
The invention provides a preparation method and a detection method of salvia miltiorrhizagranules. The preparation methodcomprises the following steps: salvia miltiorrhiza is taken as the raw material, soaked and extracted by ethanol to prepare clear paste, auxiliary materials are added, and the mixture is granulated to prepare a product. The method is simple, effective components are completely retained, the content is higher, the cost is low, the quality of the prepared salvia miltiorrhizagranules can be guaranteed, and the method is suitable for industrial production. For component detection of the detection method, salvia miltiorrhizareference crude herb, a tanshinone II A reference substance, acryptotanshinone reference substance, atanshinolreference substance, asalvianolic acid B reference substance and a protocatechualdehyde reference substance are taken as reference for effective component detection, salvianolic acid B is taken as the reference substance for content determination, the adopted component detection method has high specificity, the accuracy of content determination is high, the effective components are completely retained, the content is stable, and the quality of the prepared salvia miltiorrhizagranules is guaranteed, so that the synergistic effect of components of salvia miltiorrhiza is guaranteed, the efficacy of the salvia miltiorrhizagranulesis guaranteed, and the health of people is protected.
Owner:九寨沟天然药业股份有限公司
Taxol microcapsule as well as preparation method and detection analysis method thereof
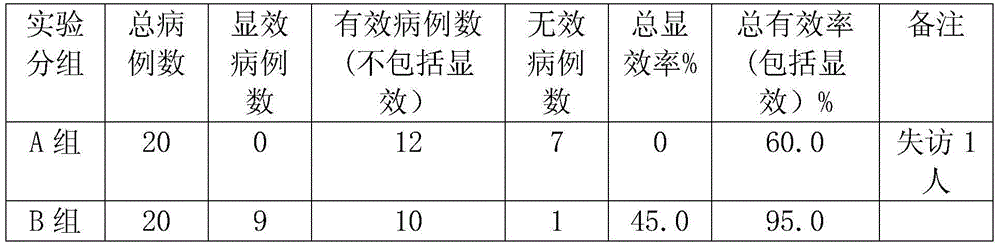
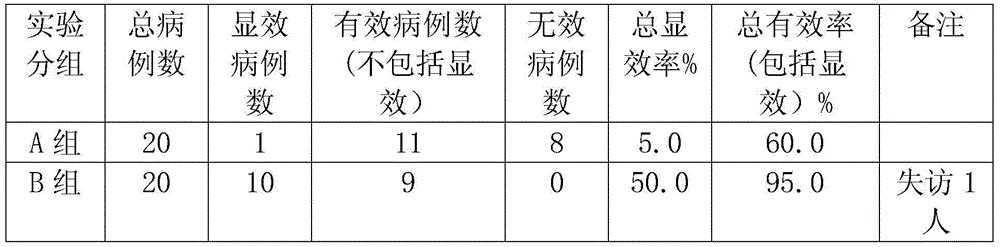
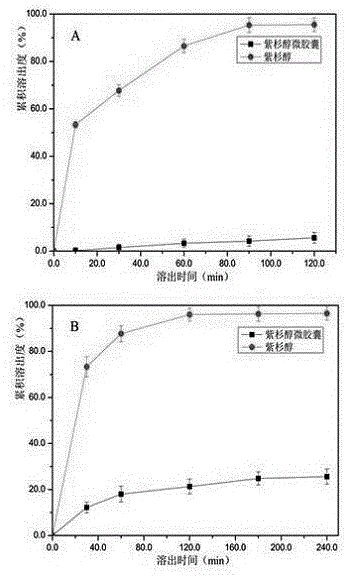
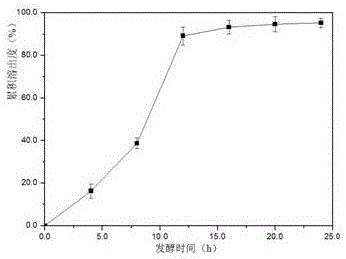
ActiveCN106562938AOvercome operabilityOvercome the shortcomings of large side effectsOrganic active ingredientsSurface/boundary effectEmbedding rateCurative effect
The invention discloses a taxol microcapsule as well as a preparation method and a detection analysis method of the taxol microcapsule. According the taxol microcapsule as well as the preparation method and the detection analysis method, gingko octenyl succinic acid modified starch is taken as a wall material, taxol is taken as a core material, the taxol microcapsule is prepared by adopting the spray drying technology, the preparation method is simple, the cost is low, and the preparation method is suitable for industrial production. According to the detection a method, taxol is taken as the reference substance, by detecting the embedding rate, the in-vitro dissolution rate and the release condition, the complete and advanced production system and the detection standard which is strong in specificity and is controllable are formed, thus the effective control over the quality of the medicine is improved, and the quality and curative effects of the taxol microcapsule are guaranteed.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Method for preparing stomach-regulating pills
ActiveCN101947298BImprove stabilityQuality is easy to controlDigestive systemAntiinfectivesAlcoholBULK ACTIVE INGREDIENT
The invention discloses a method for preparing stomach-regulating pills, which comprises the following steps of: respectively performing volatile oil extraction, water temperature extraction and alcohol extraction on different medicinal materials in the stomach-regulating formula, extracting effective parts therein, and mixing to obtain an intermediate for preparing the pills; and mixing the intermediate and a substrate, dispersing into the substrate, and performing a pill forming process to obtain the stomach-regulating pills. In the pill preparing process, each extraction link and the forming process have controllable quality, the content of active ingredients is stable, the pill preparation is carried out after the medicament intermediate is mixed fully with the substrate, and the medicament active ingredients are highly dispersed, so the prepared pills have high medicament stability.
Owner:陕西中药研究所
A compound traditional Chinese medicine preparation for treating tumors and its preparation method
ActiveCN102008650BSimple and fast operationShort cycleAntineoplastic agentsPlant ingredientsPolyvinyl alcoholPinellia
The invention provides a compound traditional Chinese medicine preparation for treating tumors and a preparation method thereof. The compound traditional Chinese medicine preparation is characterized by being prepared from the following raw materials in parts by weight: 1-4 parts of pinellia ternate, 1-4 parts of selfheal, 0.75-3.5 parts of sagittaria sagittifolia L and 0.75-5.5 parts of varech. The preparation method is characterized by smashing the raw materials into coarse powder; utilizing water or polyvinyl alcohol aqueous solution containing ethanol to extract effective ingredients from the coarse powder; and according to the specification requirements of oral preparation, extracting, concentrating and pelletizing to obtain the oral preparation. The compound traditional Chinese medicine preparation for effectively treating tumors and the preparation method of the preparation, which are provided by the invention, are verified by research on cell experiments and animal entity tumor experiments of the compound traditional Chinese medicine preparation; and better curative effect is obtained through clinical indication medications.
Owner:天津市医药科学研究所
Artemisia rupestris L pill and production method and application thereof
ActiveCN101837037BObvious anti-inflammatory and analgesic effectSolve problems such as hazardsAntipyreticAnalgesicsPolyethylene glycolRheumatism
The invention relates to an artemisia rupestris L pill and a production method and application thereof, the method comprises the following steps: step I: CommonSt.John'swortHerb and artemisia rupestris L with equal quantity are mixed and are crushed into coarse powder, ethanol is added for backflow extraction, the extracting solution is filtered, cooled and centrifuged, and then supernate is concentrated; step II: the concentrated solution is purified by macroporous absorption resin, and effluent liquid is collected in different segments, the effluent liquid is concentrated and is dried to obtain extract powder for spare; step III: the dry extract powder is mixed with polyethylene glycol according to proportion, the mixture is melted by heating, and then pills are produced by dropping. The process in the invention is stable and reliable, the contents of effective ingredients in the pills are stable; the invention solves the problem that residual organic solvent caused by the extraction of organic solvents such as petroleum ether, ethyl acetate and the like is harm to human bodies. Compared with the prior art, the method in the invention is suitable industrial production; the artemisia rupestris L pill obtained in the invention has obvious anti-inflammatory analgesic activities which are proved by experiments in pharmacodynamics and is used for treatment of rheumatism and rheumatoid arthritis.
Owner:XINJIANG QUANAN PHARMA
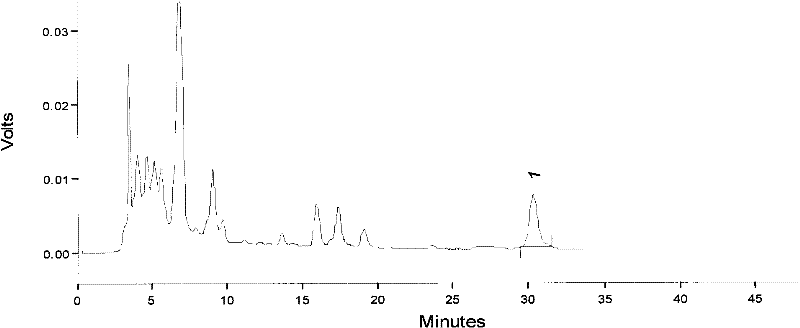
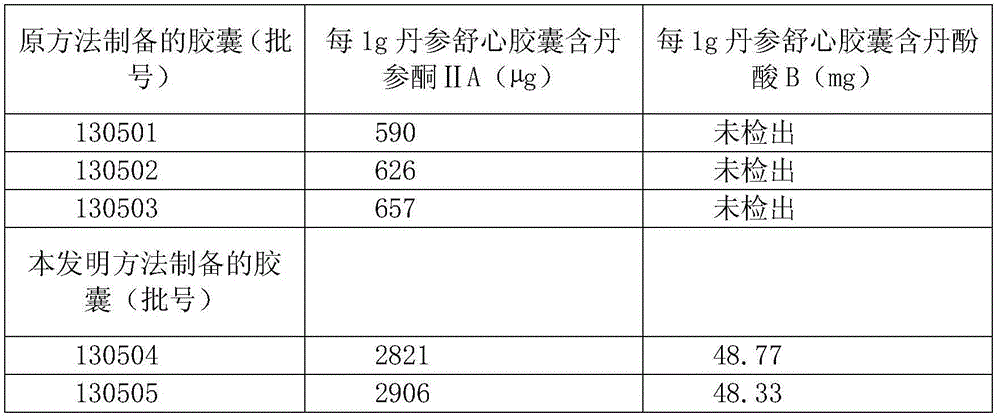
Preparation and detection methods of salvia miltiorrhiza shuxin capsule
InactiveCN104523830AAvoid lostReduce heat lossComponent separationCapsule deliverySalvianolic acid BCurative effect
The invention provides preparation and detection methods of a salvia miltiorrhiza shuxin capsule. The preparation method comprises the steps of soaking salvia miltiorrhiza serving as a raw material, and extracting by using ethanol to prepare clear paste; and adding auxiliary materials, and encapsulating to obtain the salvia miltiorrhiza shuxin capsule. The preparation method is simple, capable of completely retaining effective components and ensuring relatively-high effective component content and the quality of the salvia miltiorrhiza shuxin capsule, low in cost and also suitable for industrial production. The detection method comprises the steps of detecting effective components by taking a salvia miltiorrhiza reference medicine, a tanshinone IIA reference substance, a cryptotanshinone reference substance, a tanshinol reference substance, a salvianolic acid B reference substance and a protocatechualdehyde reference substance as references; and detecting content by taking salvianolic acid B as a reference substance. The adopted detection method for the components is strong in specificity, high in content detection accuracy, complete in effective component retention and capable of ensuring stable effective component content and the quality of the salvia miltiorrhiza shuxin capsule, so that all the components in salvia miltiorrhiza can take a synergistic effect, the curative effect is ensured, and the health of people is protected.
Owner:SICHUAN FENGCHUN PHARMA
Honeysuckle throat clearing tablets and preparation method thereof
ActiveCN102657778BEasy to takeImprove compliancePharmaceutical non-active ingredientsPill deliveryThroatAcute Pharyngitis
Owner:GENERAL HOSPITAL OF PLA
Immunity-enhancing cod-liver oil soft capsule and preparation method thereof
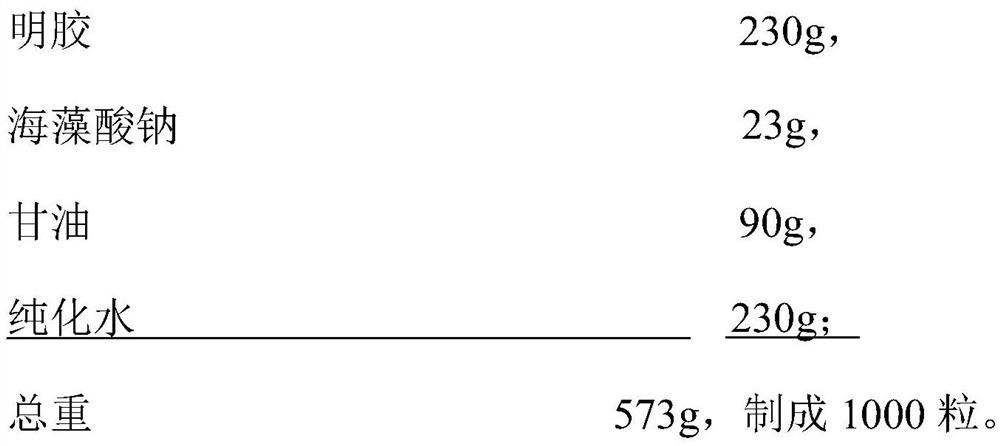
PendingCN113575939AImprove antioxidant capacityImprove palatabilityMulti-step food processesFood shapingCod liver oilSoftgel
The invention discloses an immunity-enhancing cod-liver oil soft capsule, and the capsule comprises a content and a soft capsule skin in a weight ratio of (0.66-0.68): 1; the content is prepared from the following raw materials in parts by weight: 330 to 360 parts of cod liver oil, 0.35 to 0.38 part of antioxidant, 30 to 40 parts of calcium citrate, 16 to 20 parts of grape seed oil and 2.5 to 3.3 parts of emulsifier; the soft capsule shell is prepared from the following raw materials in parts by weight: 230 to 250 parts of gelatin, 23 to 25 parts of sodium alginate, 90 to 100 parts of glycerol and 230 to 250 parts of purified water. The invention also provides a preparation method of the immunity-enhancing cod-liver oil soft capsule. The pharmaceutical composition has good oxidation resistance and preparation stability.
Owner:安徽济生元药业有限公司
Preparation method of siraitia grosvenorii compound capsules
ActiveCN104886594AUnique effectLow costNatural extract food ingredientsFood ingredient functionsFood science
The invention discloses a preparation method of siraitia grosvenorii compound capsules. The preparation method comprises the following steps: crushing raw and auxiliary materials; screening the crushed raw and auxiliary materials; mixing the screened raw and auxiliary materials; pelleting the mixed raw and auxiliary materials; coating the pelleted raw and auxiliary materials; drying the coated raw and auxiliary materials; stuffing the capsules. Each of the siraitia grosvenorii compound capsule comprises the following raw and auxiliary materials in parts by weight: 23-27% of siraitia grosvenorii compound extracts, 5-10% of persimmon powder, 30-35% of corn starch, 25-30% of maltodextrin and 5-10% of microcrystalline cellulose. The siraitia grosvenorii compound extracts use a siraitia grosvenorii extracting solution as a solvent and dried tea leaves of dried aloe leaves, dried shrub althea flowers and dried sweet basil herb as the raw materials so as to perform the extraction, the mass ratio of the dried aloe leaves to the dried shrub althea flowers and the dried sweet basil herb is 2:(0.5-2.0):(1-1.5):(0.1-1), and the material-solvent ratio is 1:(5-10); The siraitia grosvenorii compound capsules prepared by the preparation method disclosed by the invention have the oxidation resistant effect, the bacteriostasis effect and the immunity enhancing effect.
Owner:GUILIN UNIV OF ELECTRONIC TECH
A pharmaceutical composition comprising imipenem cilastatin sodium and its preparation
ActiveCN104095847BReduce central adverse reactionsStable pHAntibacterial agentsPowder deliveryImipenem/cilastatinMedicine
The invention relates to a pharmaceutical composition containing imipenem cilastatin sodium and a preparation thereof, belonging to the field of medicine. In order to overcome the technical deficiency of the large central adverse reaction of the powder injection of imipenem and cilastatin sodium in the prior art, the present invention provides a powder injection containing imipenem and cilastatin sodium through reasonable compatibility. When the powder injection is used for clinical antibacterial treatment, it can effectively reduce the central adverse reactions of imipenem cilastatin sodium compound preparation, and can significantly improve the stability of the powder injection after reconstitution, so it is suitable for development into clinical treatment drug.
Owner:CHINA NAT MEDICINES GUORUI PHARMA
Preparation method of nalmefene hydrochloride injection and nalmefene hydrochloride injection prepared with method
InactiveCN106619495AExcellent pHImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonNalmefene
The invention discloses a preparation method of a nalmefene hydrochloride injection. When the nalmefene hydrochloride injection is prepared with a conventional method, a certain quantity of citric acid or citrate auxiliary material is added to the preparation, and 5-15 g of the citric acid or citrate auxiliary material is added in each liter of the injection. During preparation, the prescription quantity of the auxiliary material is dissolved in water for injection firstly, then activated carbon is added with stirring for adsorption, and filtration is performed for impurity removal; the prescription amount of nalmefene hydrochloride is added under the nitrogen protection, after full dissolution, the solution is metered to the designated volume with water, the pH value is adjusted to 3.5-4.5, and finally, the solution is filtered by a filter, sub-packaged, filled with nitrogen, sealed and sterilized. With the adoption of the method, the expiry date of the nalmefene hydrochloride injection can be obviously prolonged, the stability is good due to adoption of a specific citric acid pH value stabilizer, the sample appearance properties, pH value, effective ingredient content, related substances and the like are relatively stable, and the medication safety is guaranteed.
Owner:威海恒基伟业信息科技发展有限公司
A method for preparing levodopa buccal tablets using broad bean flowers and broad bean sprouts
ActiveCN104173419BThe content of active ingredients is stableGreat tasteOrganic active ingredientsNervous disorderCompression moldingSide effect
The invention discloses a method for preparing levodopa buccal tablets by using broad bean flowers and broad bean sprouts, belonging to the field of biopharmaceuticals. First prepare levodopa powder and broad bean sprouts vegetable powder; then select a die, adjust the pressure and tablet weight; then mix the above-mentioned levodopa powder and broad bean sprouts vegetable powder in a certain proportion and place them in the die, and press them into tablets Obtain tablet; Put above-mentioned tablet again under ultraviolet ray and sterilize 15-20min, obtain levodopa buccal tablet. The levodopa buccal tablets prepared by the method of the present invention can not only achieve the effect of preventing and treating Parkinson's disease, reduce the side effects of chemical medicine levodopa buccal tablets, but also contain rich polysaccharides, vitamins and dietary fiber in broad bean sprouts vegetable powder etc., can improve human immunity, promote gastrointestinal peristalsis, help digestion, and prevent constipation. At the same time, the broad bean flower levodopa powder also contains polysaccharides, which improves the taste of the broad bean natural levodopa lozenges.
Owner:CROP RES INST OF FUJIAN ACAD OF AGRI SCI
Traditional Chinese medicine health care solid Xiajiu and preparation method thereof
InactiveCN108165402AKeep the traditional way of drinkingAdd funAlcoholic beverage preparationChinese drugHealth care value
The invention discloses traditional Chinese medicine health care solid Xiajiu and a preparation method thereof. The solid Xiajiu has the advantages that the traditional drinking mode of the solid Xiajiu can be still kept while traditional Chinese medicine components are introduced into the Xiajiu to give a medicinal health care value to the Xiajiu, a user can brew the solid Xiajiu containing the traditional Chinese medicine components and drink the Xiajiu in a sucking way instead of directly drinking Xiajiu finished liquor containing the traditional Chinese medicine components when drinking the Xiajiu, the Xiajiu and the method enhance enjoyment and cultural experience and feeling of consumers while giving medicinal liquor taste and medicinal health care value to the Xiajiu, and are beneficial to transmission of Xiajiu culture and promotion of Xiajiu products.
Owner:四川省宕府王食品有限责任公司
Method and system for controlling addition of polyester waste washing liquid
InactiveCN110843154AImprove and stabilize qualityLow PVC contentPlastic recyclingCleaning using liquidsPolyesterWaste material
The invention provides a method for controlling addition of polyester waste washing liquid. The method comprises the following steps: preparing the polyester waste washing liquid, wherein the concentration of hydroxyl ions in the washing liquid is marked as R; mixing the washing liquid with water to obtain washing liquid; setting the concentration of the hydroxyl ions in the washing liquid according to the type of polyester waste and the product requirements of a regenerated polyester bottle; and cleaning the polyester waste by using the washing liquid, and periodically detecting the concentration of the hydroxyl ions in the washing liquid in the cleaning process. According to the method, the concentration of the hydroxyl ions in the washing liquid and the preset concentration of the hydroxyl ions are periodically detected, and a quantitative amount of washing liquid can be added according to the detected concentration of the hydroxyl ions in the washing liquid, so that the content ofeffective components in the washing liquid is stable, and the quality of the regenerated polyester bottle is effectively improved and stabilized.
Owner:ZHEJIANG HAILI ENVIRONMENTAL TECH CO LTD
Method for detoxification of velvet bean and application of byproduct in textile dyeing
PendingCN112900116AImprove utilization efficiencyHigh content of active ingredientsDyeing processBiotechnologyPulp and paper industry
The invention discloses a method for detoxification of velvet bean and application of byproduct in textile dyeing. The method comprises the following steps of 1, carrying out smashing treatment on velvet bean; 2, performing detoxication of the smashed velvet bean; 4, performing continuous vat detoxification; and 5, jumping to the step 3. According to the invention, the pollution is reduced and the cost is low; while the production efficiency is improved, chemical pollution is not increased, the pure natural properties of a main product filtrate and a byproduct filtrate are still kept; the environment is protected, the cost of auxiliaries is not increased, and the natural components of feed raw materials are not changed; the main component of the cooled filtrate is a levodopa solution; and the byproduct is directly used for textile dyeing, so that waste utilization is realized, and the added value of a textile is improved.
Owner:杭州植彩纺织科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com