Preparation method of the B-Al-ZSM-11 zeolite catalyst and application thereof
A zeolite catalyst and zeolite technology are applied in molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effects of slowing down further side reactions, improving diffusion performance, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] At room temperature, 126.2g of tetrabutylammonium bromide and 439.2g of silica sol (30wt%) were dissolved in 100mL of water; under stirring, aqueous sodium hydroxide solution (16.4g of sodium hydroxide+200mL of water) was added dropwise, and stirred After 1 hour, add the aqueous solution of boric acid and sodium aluminate (54.4g boric acid + 0.3g sodium aluminate + 602ml water) and continue to stir for 1 hour, put it in a kettle, crystallize at 160°C for 3 days, filter, wash, and dry overnight at 100°C , B-Al-ZSM-11 was obtained after calcination at 540℃ for 6h. Raw materials used, in terms of molar ratio of pure substances, SiO in silicon source 2 : B in boron source 2 o 3 : Al in aluminum source 2 o 3 : TBA in the templating agent + : OH in alkali source - : Inorganic salt: H 2 O=1:0.2:0.00083:0.18:0.19:0:30.6.
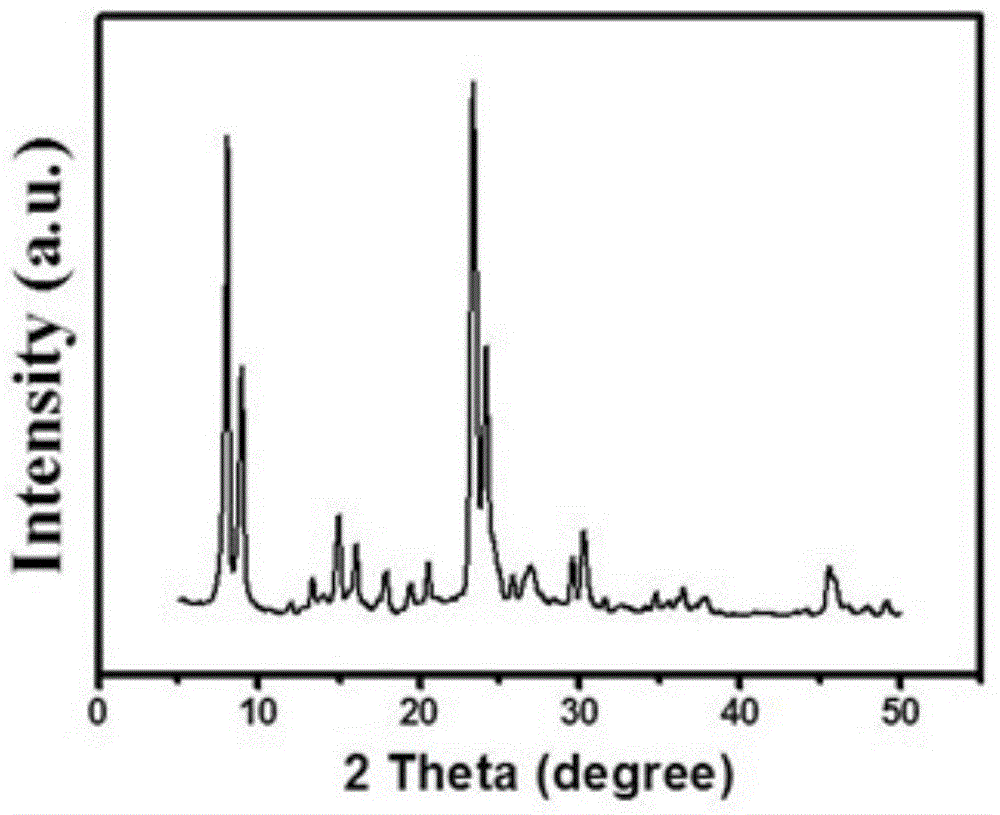
[0031] figure 1 is the XRD pattern of the sample, it can be seen from the figure that the sample has a MEL structure;
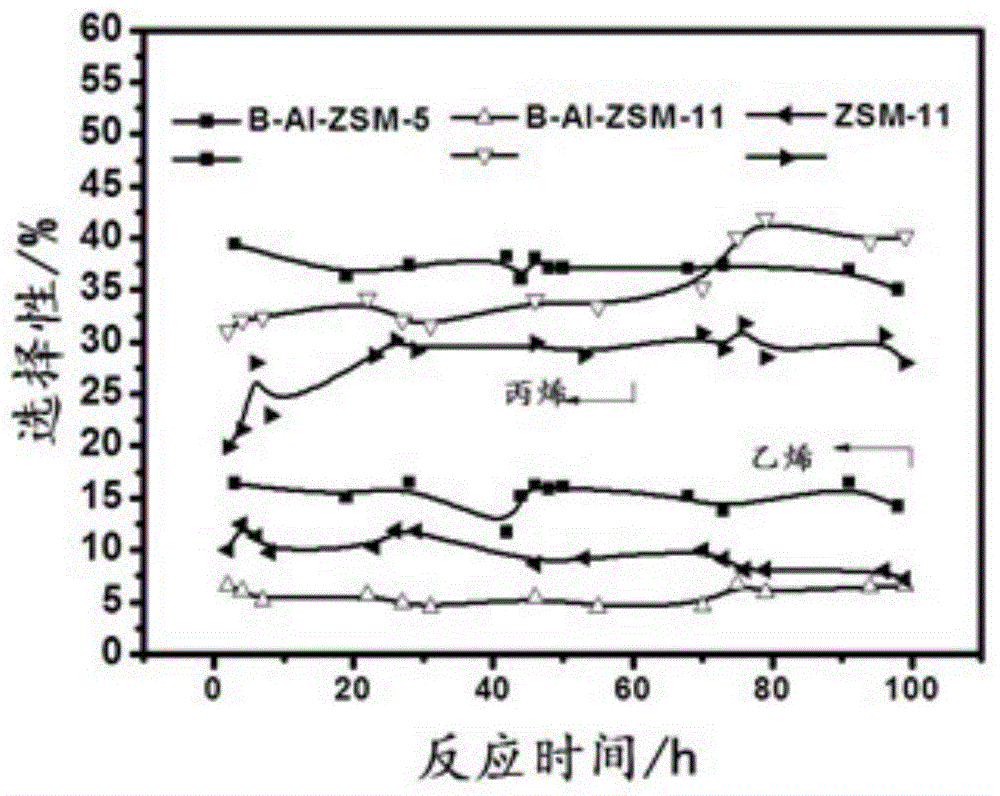
[0032] figure 2 It is the...
Embodiment 2
[0034] At room temperature, 86g of tetrabutylammonium bromide and 330.4g of silica sol (30wt%) were dissolved in 400mL of water; under stirring, aqueous sodium hydroxide solution (10.6g of sodium hydroxide+200mL of water) was added dropwise thereto, and stirred for 3 hour, add the aqueous solution of boric acid and sodium aluminate (41.6g boric acid+0.48g aluminum sulfate+58g sodium chloride+500ml water) and continue to stir for 3 hours, put in a kettle, crystallize at 160°C for 3 days, filter, wash, 100 °C dried overnight, and then calcined at 580 °C for 1 h to obtain B-Al-ZSM-11. Raw materials used, in terms of molar ratio of pure substances, SiO in silicon source 2 : B in boron source 2 o 3 : Al in aluminum source 2 o 3 : TBA in the templating agent + : OH in alkali source - : Inorganic salt: H 2 O=1:0.2:0.00083:0.16:0.16:0.6:44.8.
Embodiment 3
[0036] At room temperature, 35.1g of tetrabutylammonium bromide and 131.8g of white carbon black were dissolved in 200mL of water; under stirring, aqueous sodium hydroxide solution (8.6g of sodium hydroxide + 100mL of water) was added dropwise thereto, and stirred for 2 hours, Add the aqueous solution of boric acid and sodium aluminate (2.1g sodium borate + 0.18g sodium aluminate + 95ml water) and continue to stir for 2 hours, put it in a kettle, crystallize at 160°C for 3 days, filter, wash, dry overnight at 100°C, 560 B-Al-ZSM-11 was obtained after calcination at ℃ for 3h. Raw materials used, in terms of molar ratio of pure substances, SiO in silicon source 2: B in boron source 2 o 3 : Al in aluminum source 2 o 3 : TBA in the templating agent + : OH in alkali source - : Inorganic salt: H 2 O=1:0.005:0.0005:0.05:0.1:0:10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com