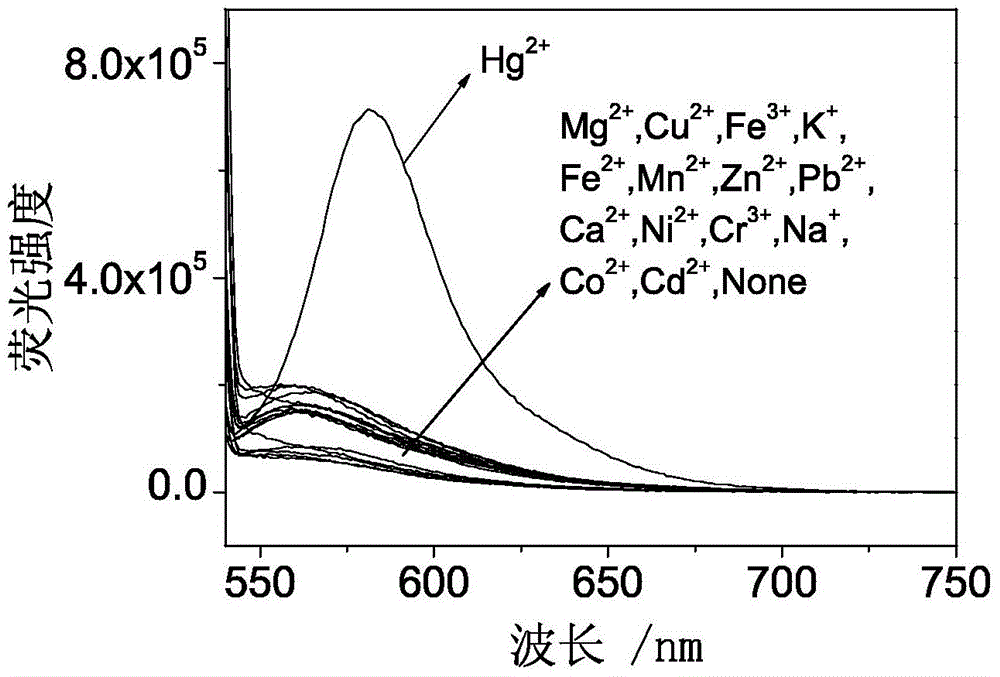
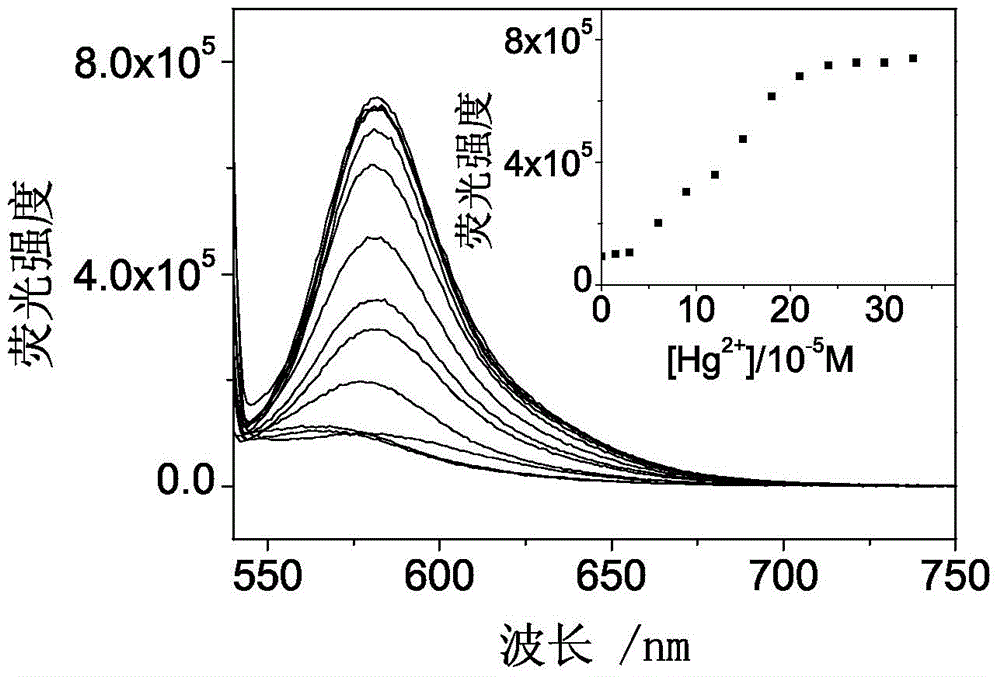
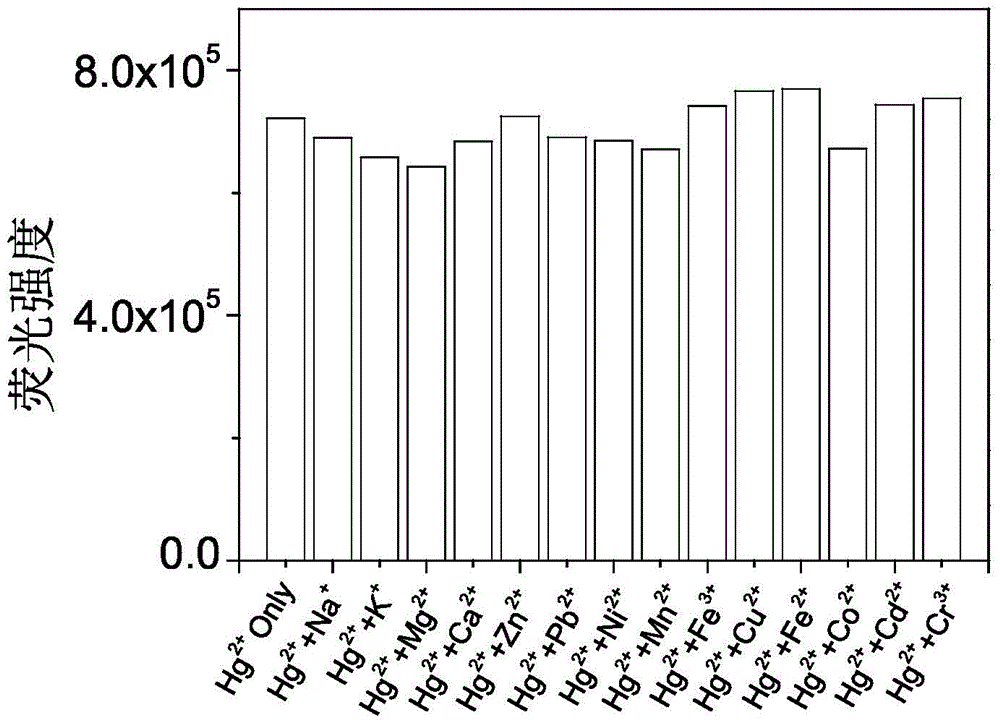
Fluorescence probe based on RB (rhodamine B), TEPA (tetraethylenepentamine) and PITC (phenyl isothiocyanate) as well as preparation method and application of fluorescence probe
A technology of tetraethylenepentamine and phenylthiourea, which is applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of inability to detect, poor selectivity, poor water solubility, etc., to improve detection sensitivity, Good practicability and the effect of improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment one: Hg 2+ Preparation of probe RPTU.
[0033] Using ethanol as a solvent, dissolve 0.2 g (0.42 mmol) rhodamine B in ethanol, then add 956 μL (5.04 mmol) tetraethylenepentamine dropwise, heat to 80°C for 12 h, stop the reaction, and use The solvent was removed by a rotary evaporator, washed three times with water, and dried to obtain a yellow solid, which is the intermediate RTPA. The yield was 90.8%.
[0034] Dissolve 50 mg (0.081 mmol) of intermediate RTPA and 76.8 μL (0.648 mmol) of phenyl isothiocyanate in anhydrous tetrahydrofuran, stir the reaction at 60 °C for 6 h, remove the solvent by rotary evaporation, separate on a silica gel column, and elute The agent is a mixture of ethyl acetate and petroleum ether (v / v, 1 / 5), and a white powder product is obtained, which is rhodamine B-phenylthiourea derivative (Hg 2+ probe) RPTU with a yield of 13.6%.
[0035] IR (KBr) cm -1 : 3460, 3209 (NH), 2924, 2854 (CH 3 , CH 2 ), 1639 (C=O), 1601, 1543, 1493 (A...
Embodiment 2
[0037] Embodiment two: Hg 2+ Preparation of probe RPTU.
[0038] With isopropanol as solvent, 0.2 g (0.42 mmol) Rhodamine B was dissolved in isopropanol, then 796.7 μL (4.2 mmol) tetraethylenepentamine was added dropwise, heated to 80 °C for 10 h, Stop the reaction, remove the solvent with a rotary evaporator, wash with water three times, and dry to obtain a yellow solid, which is the intermediate RTPA. The yield was 81.9%.
[0039] Dissolve 50 mg (0.081 mmol) of intermediate RTPA and 96.0 μL (0.81 mmol) of phenylisothiocyanate in anhydrous acetonitrile, stir the reaction at 20 °C for 6 h, remove the solvent by rotary evaporation, separate on a silica gel column, and elute The agent is a mixture of ethyl acetate and petroleum ether (v / v, 1 / 5), and a white powder product is obtained, which is rhodamine B-phenylthiourea derivative (Hg 2+ probe) RPTU with a yield of 21.8%.
Embodiment 3
[0040] Embodiment three: Hg 2+ Preparation of probe RPTU.
[0041] With n-butanol as solvent, 0.2 g (0.42 mmol) rhodamine B was dissolved in n-butanol, then 1.2 mL (6.3 mmol) tetraethylenepentamine was added dropwise, heated to 90°C for 10 h, Stop the reaction, remove the solvent with a rotary evaporator, wash with water three times, and dry to obtain a yellow solid, which is the intermediate RTPA. The yield was 78.2%.
[0042] Dissolve 50 mg (0.081 mmol) of intermediate RTPA and 115.2 μL (0.972 mmol) of phenylisothiocyanate in anhydrous acetonitrile, stir the reaction at 30 °C for 6 h, remove the solvent by rotary evaporation, separate on a silica gel column, and elute The agent is a mixture of ethyl acetate and petroleum ether (v / v, 1 / 5), and a white powder product is obtained, which is rhodamine B-phenylthiourea derivative (Hg 2+ probe) RPTU with a yield of 36.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com