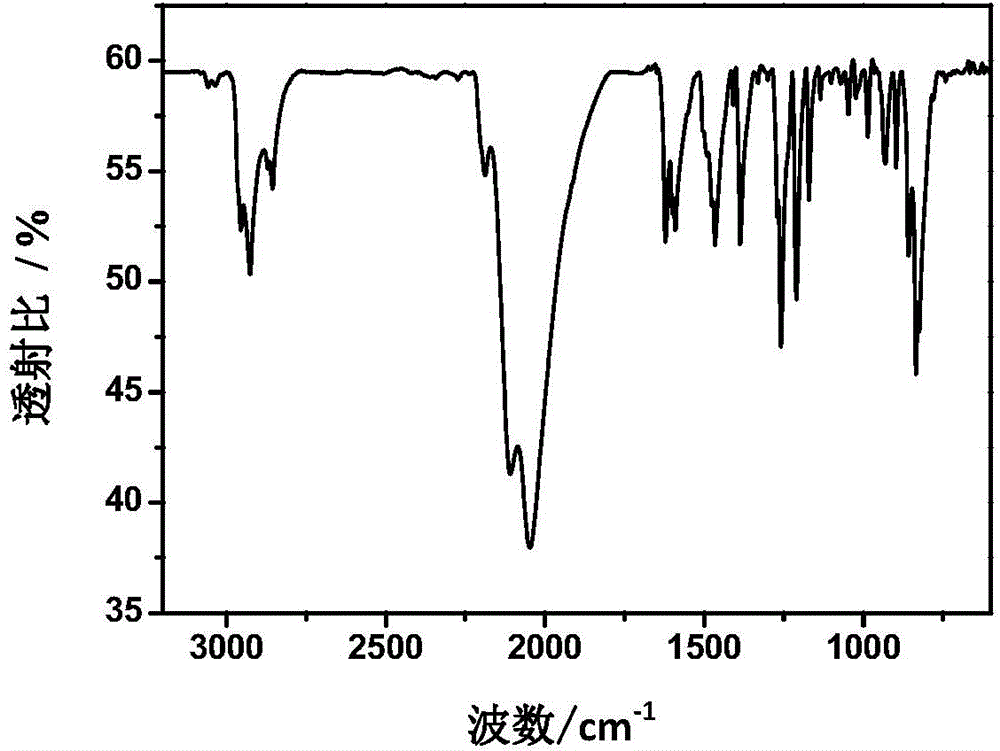
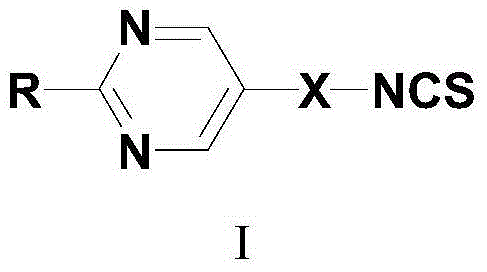
Pyrimidine liquid crystal compound with high birefringence and preparation method thereof
A technology of compounds and intermediates, which is applied in the field of pyrimidine liquid crystal compounds and their preparation, can solve problems such as high viscosity, melting point and clearing point, and affect liquid crystal mixing and compatibility, and achieve weak interaction, reduced thickness, and broad The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of compound Ia
[0045]
[0046] Take R as X is The formula I compound of the present invention introduces the synthetic method of the pyrimidine liquid crystal compound containing isothiocyanate group as an example:
[0047] 1. Synthesis of intermediate m1-1:
[0048]
[0049]Add 2.26g (12mmol) of 4-pentyloxyphenylacetylene and 2.84g (10mmol) of 2-iodo-5-bromopyrimidine into a 250ml three-necked flask, add 50ml of tetrahydrofuran and 50ml of triethylamine to completely dissolve the raw materials, and pass argon After gas sonication for 30 minutes, 105 mg of tetrakis(triphenylphosphine) palladium, 57 mg of cuprous iodide, and 75 mg of triphenylphosphine were added, and the mixture was incubated at 35° C. under argon gas for 8 hours. After the reaction, the solvent was distilled off and purified by column chromatography. 2.79 g of white solid m1-1 was obtained with a yield of 81%.
[0050] 2. Synthesis of intermediate m2-1:
[00...
Embodiment 2
[0059] Embodiment 2: the synthesis of compound Ib
[0060]
[0061] Take R as -O-C 5 h 11 , X is The formula I compound of the present invention introduces the synthetic method of the pyrimidine liquid crystal compound containing isothiocyanate group as an example:
[0062] 1. Synthesis of intermediate m1-2:
[0063]
[0064] Add 5.22g (30mmol) of 5-bromo-2-hydroxypyrimidine into a 100mL three-necked flask, then add 30mL of N-N-dimethylformamide (DMF), and stir. Then 12ml of deionized water, 0.25g of tetrabutylammonium bromide, and 6.21g (45mmol) of potassium carbonate were added respectively. The temperature was raised to 65° C., and then 6 g (40 mmol) of n-pentane bromide was added dropwise. After the dropwise addition was completed, the reaction was carried out under temperature control for 4 hours. After the reaction, wash with water, extract, distill off the solvent, and purify by column chromatography. 5.71 g of white solid m1-2 was obtained, with a yield o...
Embodiment 3
[0074] Embodiment 3: the synthesis of compound Ic
[0075]
[0076] Take R as -O-C 5 h 11 , X is The formula I compound of the present invention introduces the synthetic method of the pyrimidine liquid crystal compound containing isothiocyanate group as an example:
[0077] 1. Synthesis of intermediate m1-2:
[0078]
[0079] Add 4.35g (25mmol) of 5-bromo-2-hydroxypyrimidine into a 100mL three-necked flask, then add 25mL of N-N-dimethylformamide (DMF), and stir. Then 10 mL of deionized water, 0.21 g of tetrabutylammonium bromide, and 5.17 g (37.5 mmol) of potassium carbonate were added respectively. The temperature was raised to 65° C., and then 5 g (33 mmol) of n-pentane bromide was added dropwise. After the dropwise addition was completed, the reaction was carried out under temperature control for 4 hours. After the reaction, wash with water, extract, distill off the solvent, and purify by column chromatography. Obtained 4.82g white solid m1-2, yield 79%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com