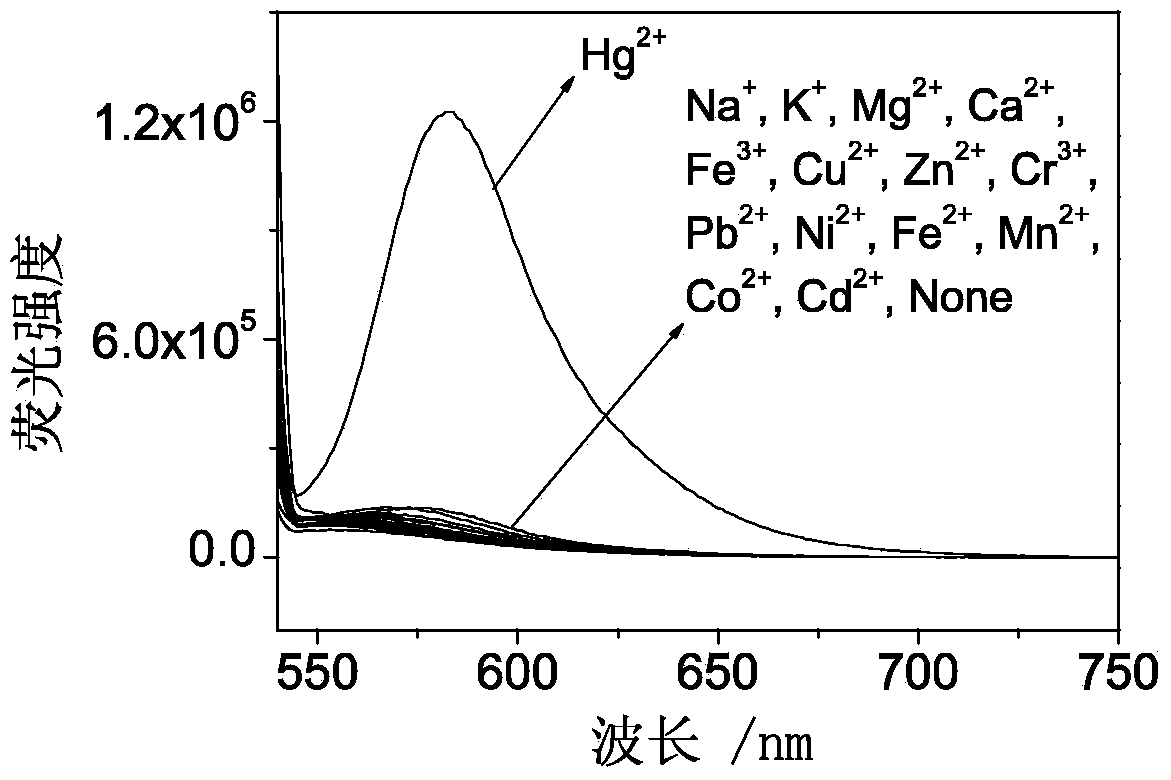
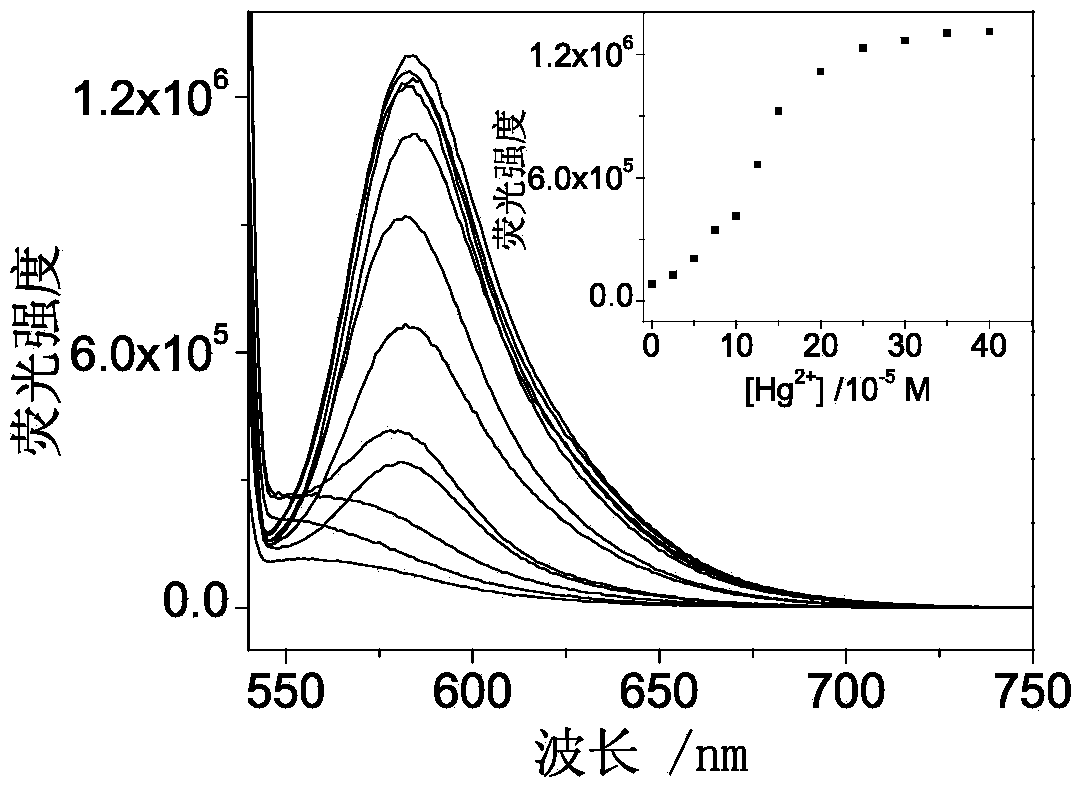
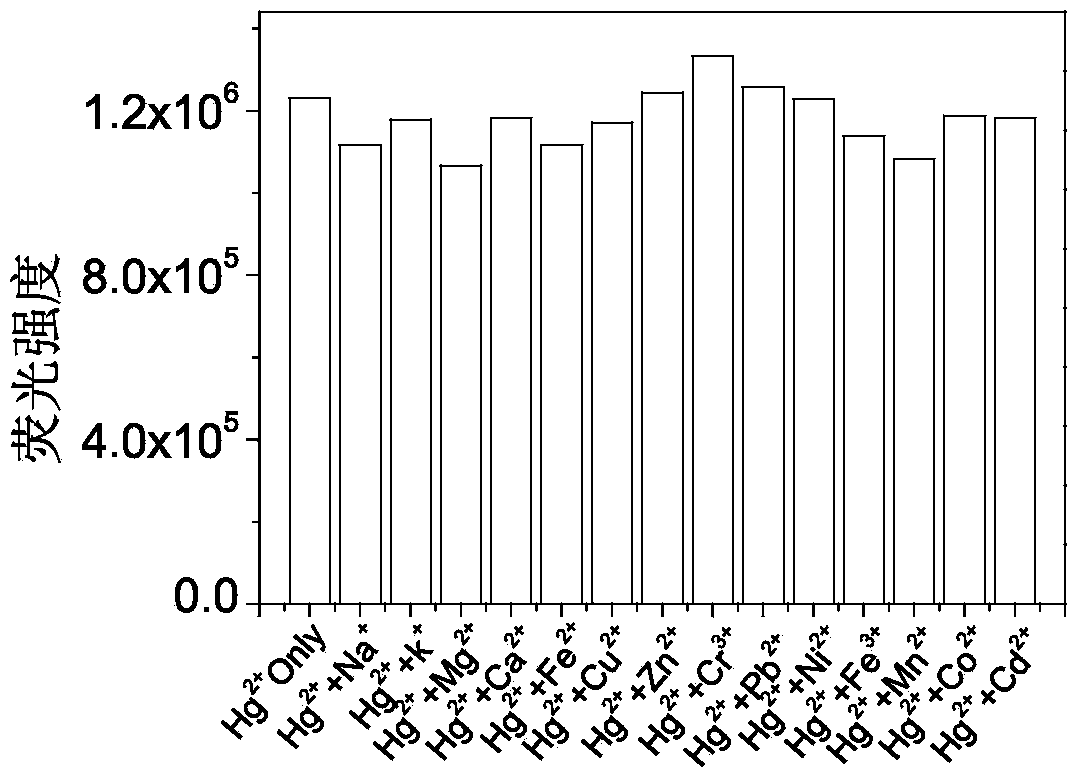
Fluorescent probe synthesized by rhodamine B, triethylene tetramine and phenyl isothiocyanate and preparing method and application thereof
A technology of triethylenetetramine and phenylthiourea, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of difficult synthesis, undetectable, poor selectivity, etc., and achieve a simple and easy synthesis method , Improve the detection sensitivity, improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment one: Hg 2+ Preparation of probe RTTU.
[0034] Using ethanol as a solvent, dissolve 0.5 g (1.05 mmol) rhodamine B in ethanol, then add 1.57 mL (10.5 mmol) triethylenetetramine dropwise, heat to 80°C for 24 h, stop the reaction, and evaporate by rotary evaporation CH 2 Cl 2 and H 2 O extracted 3 times, the organic layer was collected, and the CH was removed by rotary evaporation 2 Cl 2 , to obtain RTTA as a yellow solid. The yield was 86.6%.
[0035] Dissolve 0.1 g (0.175 mmol) of intermediate RTTA and 124.5 μL (1.05 mmol) of phenyl isothiocyanate in acetonitrile, stir the reaction at 80 °C for 48 h, cool to 20 °C, and precipitate a white solid. Stir for 2 h, let stand for 12 h, filter, wash with acetonitrile, and dry to obtain a white powder product, which is rhodamine B-phenylthiourea derivative (Hg 2+ probe) RTTU with a yield of 19.5%.
[0036] IR (KBr) cm -1 : 3245(NH), 1616, 1544, 1515 (ArH), 2969, 2929 (CH 3 , CH 2 ), 1675 (C=O), 1379, 1450 (...
Embodiment 2
[0038] Embodiment two: Hg 2+ Preparation of probe RTTU.
[0039] Using ethanol as a solvent, dissolve 0.5 g (1.05 mmol) rhodamine B in ethanol, then add 1.88 mL (12.6 mmol) triethylenetetramine dropwise, heat to 70°C for 30 h, stop the reaction, and evaporate by rotary evaporation CH 2 Cl 2 and H 2 O extracted 3 times, the organic layer was collected, and the CH was removed by rotary evaporation 2 Cl 2 , to obtain RTTA as a yellow solid. The yield was 84.3%.
[0040] Dissolve 0.1 g (0.175 mmol) of intermediate RTTA and 207.5 μL (1.75 mmol) of phenyl isothiocyanate in acetonitrile, stir the reaction at 70 °C for 72 h, cool to 20 °C, and precipitate a white solid. Stir for 2 h, let stand for 2 days, filter, wash with acetonitrile, and dry to obtain a white powder product, which is rhodamine B-phenylthiourea derivative (Hg 2+ probe) RTTU with a yield of 23.3%.
Embodiment 3
[0041] Embodiment three: Hg 2+ Preparation of probe RTTU.
[0042] Using isopropanol as solvent, dissolve 0.5 g (1.05 mmol) rhodamine B in isopropanol, then add 1.88 mL (12.6 mmol) triethylenetetramine dropwise, heat to 80°C for 24 h, stop Reaction, after removal of solvent by rotary evaporation, CH 2 Cl 2 and H 2 O extracted 3 times, the organic layer was collected, and the CH was removed by rotary evaporation 2 Cl 2 , to obtain RTTA as a yellow solid. The yield was 76.9%.
[0043] Dissolve 0.1 g (0.175 mmol) of intermediate RTTA and 249 μL (2.1 mmol) of phenyl isothiocyanate in tetrahydrofuran, stir the reaction at 60 °C for 72 h, cool to 20 °C, and precipitate a white solid. Stir for 2 h, let stand for 12 h, filter, wash with acetonitrile, and dry to obtain a white powder product, which is rhodamine B-phenylthiourea derivative (Hg 2+ probe) RTTU with a yield of 32.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com