Preparation method of lithium ion battery positive electrode composite material and precursor thereof
A lithium-ion battery and composite material technology, which is applied in the field of new energy materials and preparation, can solve the problems of not meeting the requirements of environmental protection, low utilization rate of by-products, and many steps in the synthesis process, so as to achieve easy operation and increase product addition. The effect that the value and synthesis method are simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) 100 g of nickel chloride hydrate, cobalt chloride hydrate, and manganese chloride hydrate are weighed in a molar ratio of 1:1:1 and put into a reactor, heated to 90° C.;
[0031] (2) Pass liquid ammonia under the protection of inert gas argon or nitrogen, supplement 62.7g water, add slowly from the dropping funnel, react while stirring, the flow rate of liquid ammonia is controlled at 0.04L / min;
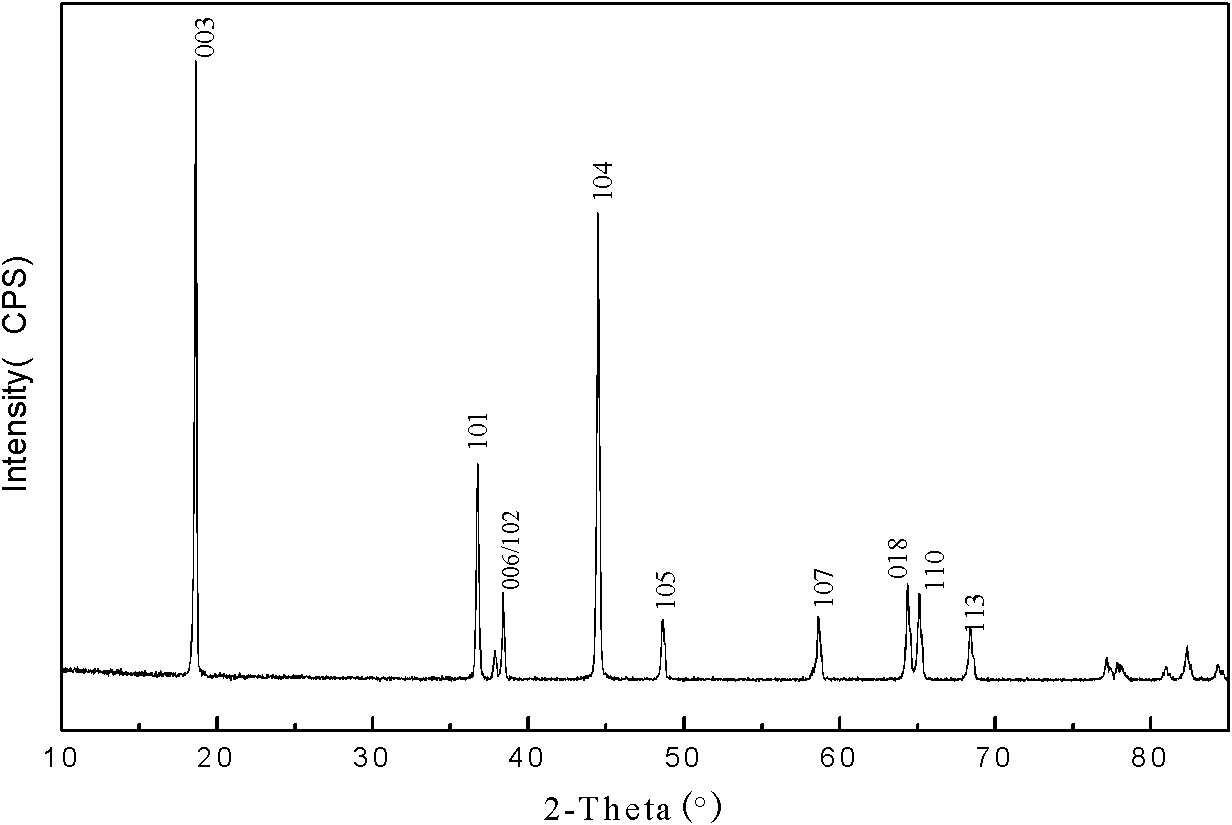
[0032] (3) After the reaction is complete, change to a distillation unit. Water is used as a circulating coolant in the distillation unit, and the gas receiving unit adopts water to absorb hydrogen chloride and ammonia. Heating with an electric heating mantle, the temperature is controlled at 340°C, the ammonium salt is distilled off, the solid is taken out, and dried to obtain an amorphous ternary cathode material precursor.
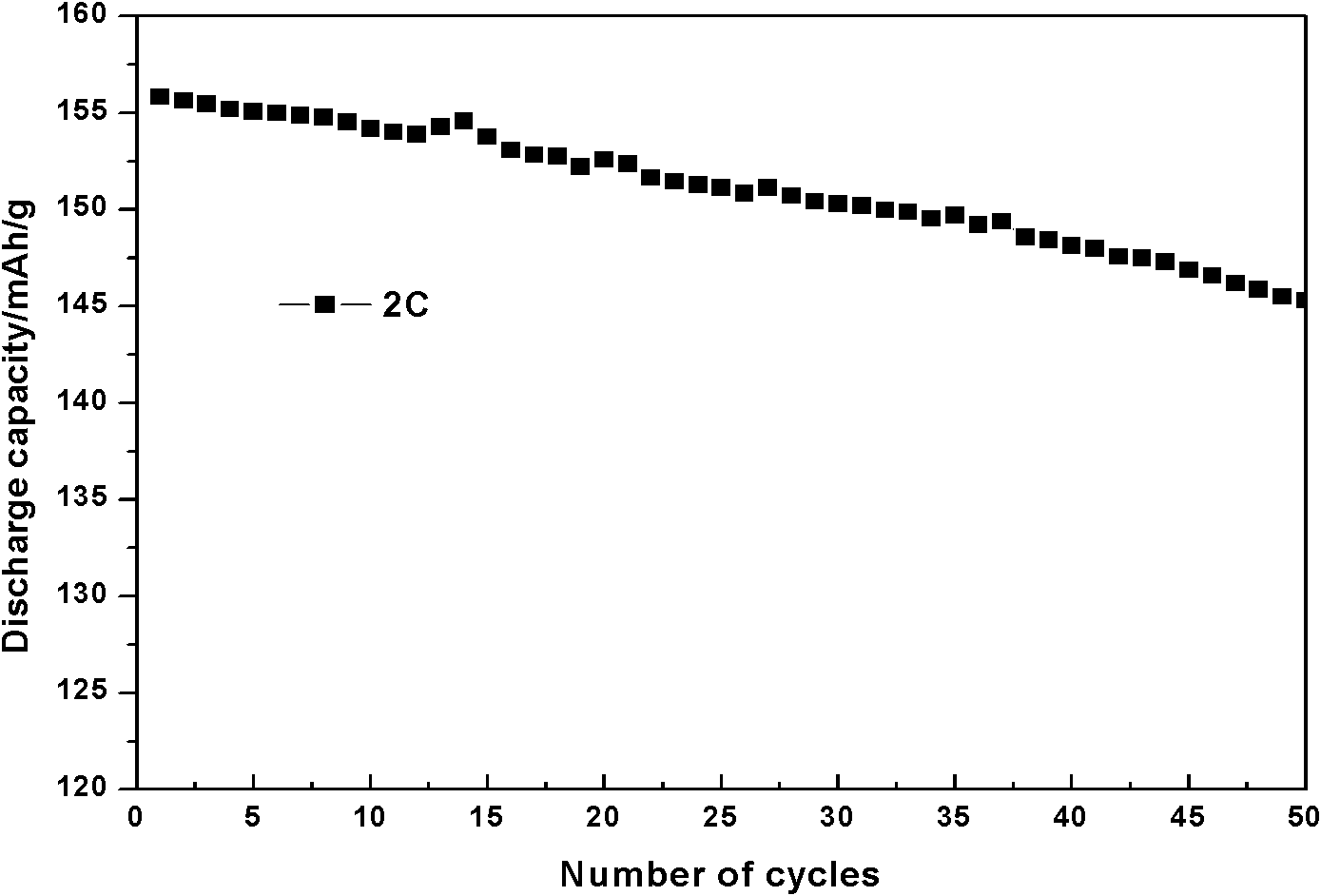
[0033] (4) The precursor and lithium carbonate are mixed in a ratio of 1:1:1 by metal ion and lithium ion molar ratio, mixed evenly in a planetary ...
Embodiment 2
[0036] (1) Weigh nickel chloride hydrate, cobalt chloride hydrate and manganese chloride hydrate with a molar ratio of 1:1:1 and put 100 g in total into a three-necked flask, and heat to 120° C.;
[0037] (2) Pass liquid ammonia under the protection of inert gas argon or nitrogen, react while stirring, and the flow rate of liquid ammonia is controlled at 0.08L / min;
[0038] (3) After the reaction is complete, change to a distillation unit. Water is used as a circulating coolant in the distillation unit, and the gas receiving unit adopts water to absorb hydrogen chloride and ammonia. Heating with an electric heating mantle, the temperature is controlled at 360°C, the ammonium salt is distilled off, the solid is taken out, and dried to obtain the precursor of the amorphous ternary cathode material.
[0039] (4) Mix the precursor and lithium carbonate according to the ratio of metal ion to lithium ion molar ratio of 1:118, mix the materials evenly in a planetary ball mill, sinter...
Embodiment 3
[0042] (1) 100 g of nickel chloride hydrate, cobalt chloride hydrate and manganese chloride hydrate were weighed in a molar ratio of 1:1:1 and put into a three-necked flask, heated to 110° C.;
[0043] (2) Pass liquid ammonia under the protection of inert gas argon or nitrogen, react while stirring, and the flow rate of liquid ammonia is controlled at 0.05L / min.
[0044] (3) After the reaction is complete, the distillation unit is changed into a distillation unit. In the distillation unit, water is used as a circulating coolant, and the gas receiving unit adopts water to absorb ammonium chloride. Heating with an electric heating mantle, the temperature is controlled at 355°C, the ammonium salt is distilled off, the solid is taken out, and dried to obtain the precursor of the amorphous ternary cathode material.
[0045] (4) Mix the precursor and lithium carbonate according to the ratio of metal ion to lithium ion molar ratio of 1:1.22, mix the materials evenly in a planetary ba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| D50 | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| D50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com