Method for synthesizing dipeptide with glutamic acid as first amino acid residue
A glutamic acid and amino acid technology, applied in the direction of peptides, etc., can solve the problems of restricted application, inability to crystallize the product, and low total yield, and achieve the effects of facilitating industrial scale production, shortening the process flow, and simplifying the synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
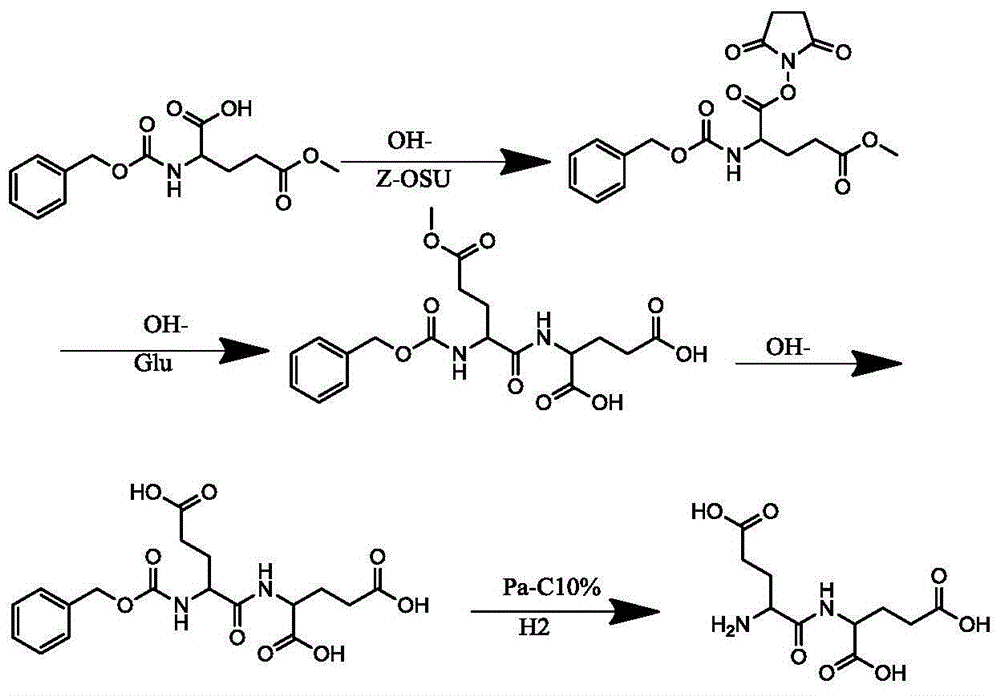
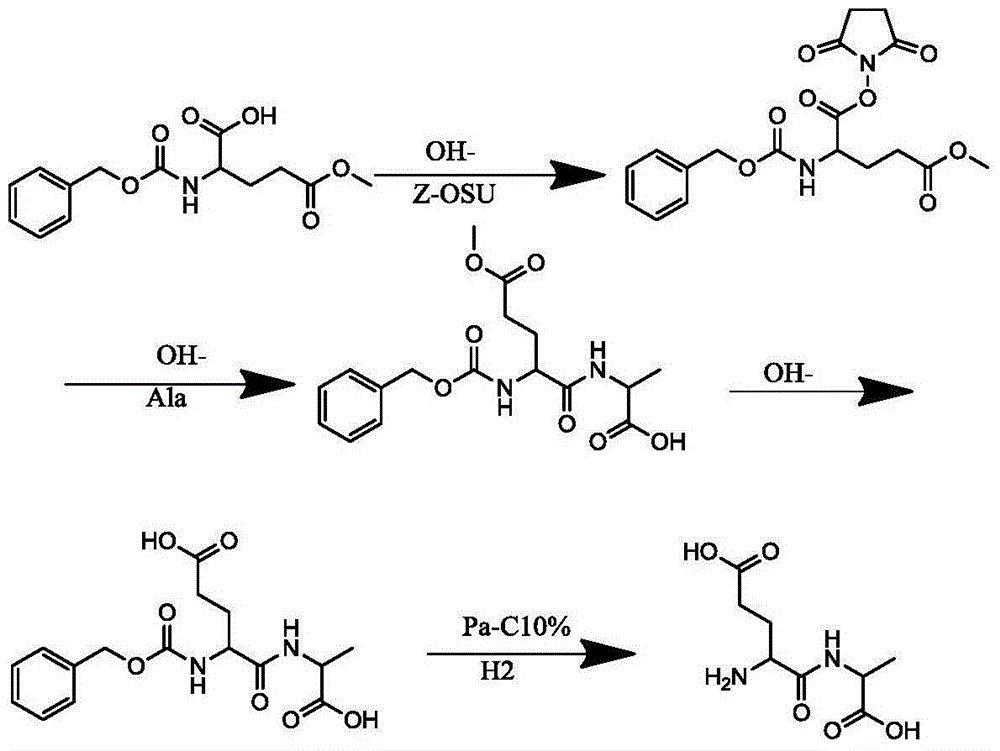
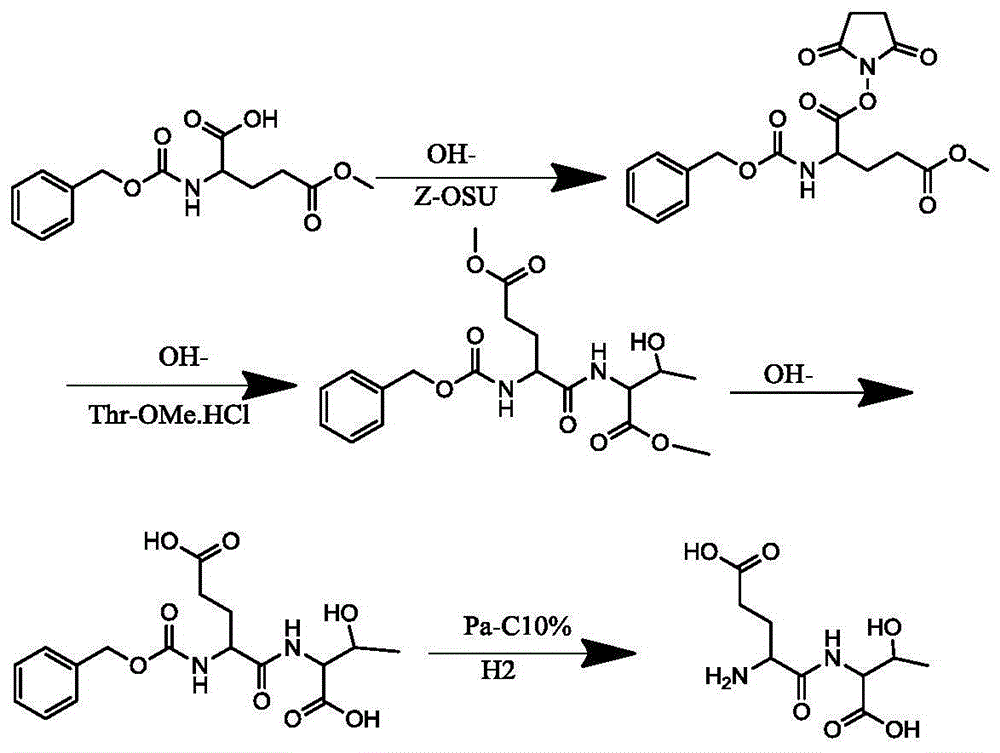
[0018] Add 1000L water and 200KG 5-methyl glutamate to a 2000L reaction tank, and add sodium carbonate in portions to adjust the pH value until the raw materials are dissolved, and the pH value should be between 8-9. Add the protective reagent Z-OSU at 0-10°C and react for about 10 hours. During the reaction, TLC monitors the reaction. After the reaction of 5 methyl glutamate is over 95%, adjust the pH to 2-3 with 6N hydrochloric acid solution, and add 600L ethyl acetate Ester extraction, separate the organic layer, TLC monitors the extraction of the product in the water, 25 kg of anhydrous sodium sulfate is dried for 6 hours, filtered and concentrated to obtain 330 kg of solid benzyloxycarbonylglutamic acid 5-methyl ester Z-Glu (OMe), HPLC: 98.32%.
Embodiment 2
[0020] Add 2000L of methylene chloride in a 3000L reaction tank, add 330 kg of benzyloxycarbonylglutamic acid 5 methyl ester Z-Glu (OMe), then add 154.4 kg of N-hydroxysuccinimide HOSU, add 276.5 kg Kilograms of dicyclohexylcarbodiimide DCC, react at 10-25 degrees for 8 hours, wait for Z-Glu (OMe) to react to more than 95%, filter and concentrate to obtain benzyloxycarbonyl glutamic acid 5 methyl ester 1 succinimide ester Z -Glu(OMe)-OSU white solid 381.5KG, HPLC: 97.1%.
Embodiment 3
[0022] Add 1140L water in the reaction tank of 2000L, add 138.8 kilograms of glutamic acid, add 200 kilograms of sodium carbonate, 114 kilograms of tetrahydrofuran, add 5 methyl benzyloxycarbonyl glutamic acid 1 succinimide ester Z-Glu (OMe )-OSU solid 381.5 kg, the temperature is controlled at 25-30 degrees for 3-5 hours, TLC confirms that the Z-Glu(OMe)-OSU reaction is above 98%, add 38L of 10N sodium hydroxide solution, and react at 40-45 degrees After 5-6 hours, TLC monitored the reaction, added 6N hydrochloric acid to adjust the pH value to 2-3, and precipitated a solid to obtain (full Chinese chemical name) benzyloxycarbonyl glutamyl glutamic acid, centrifuge, and dry to obtain 335KG.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com