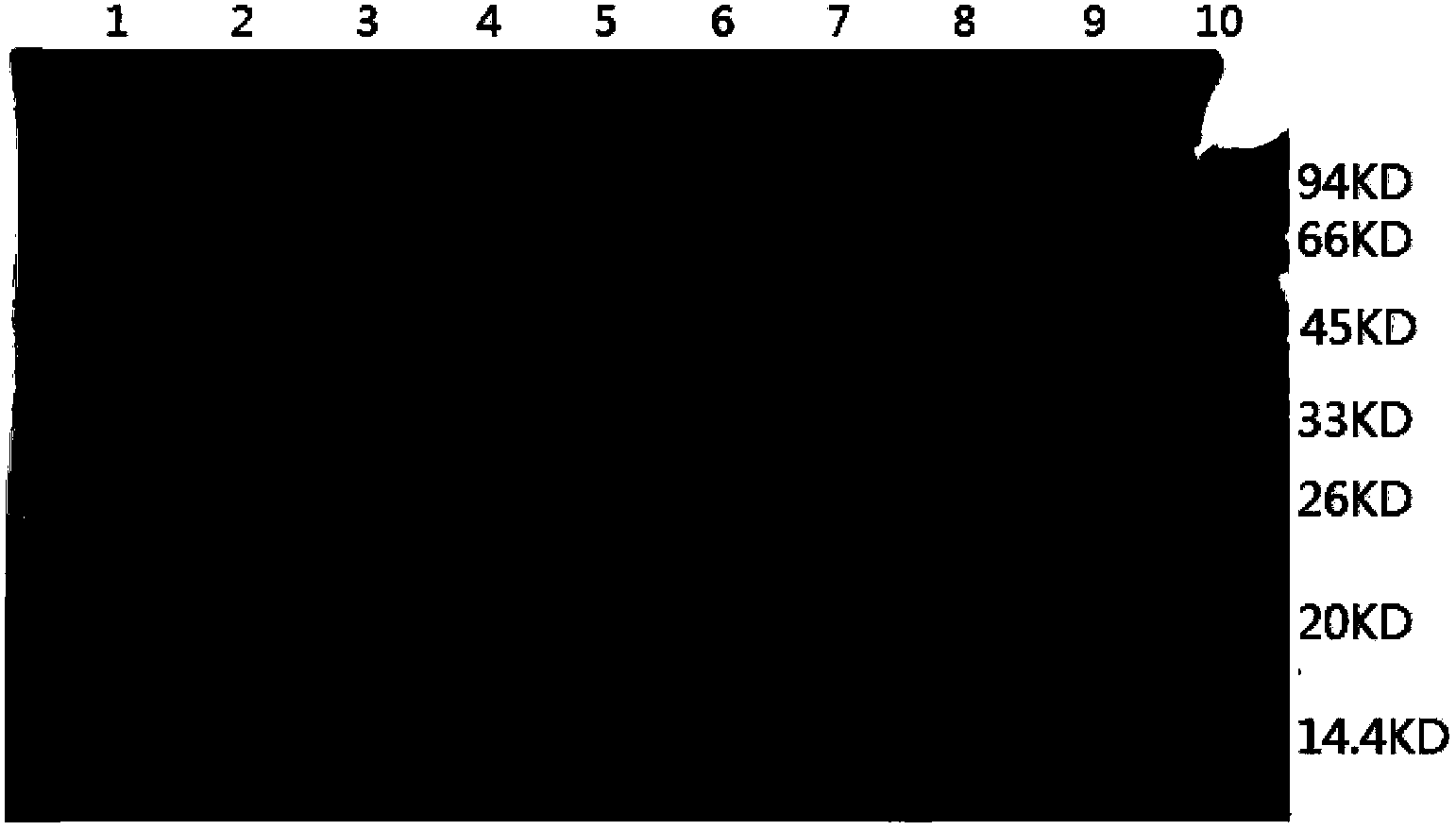
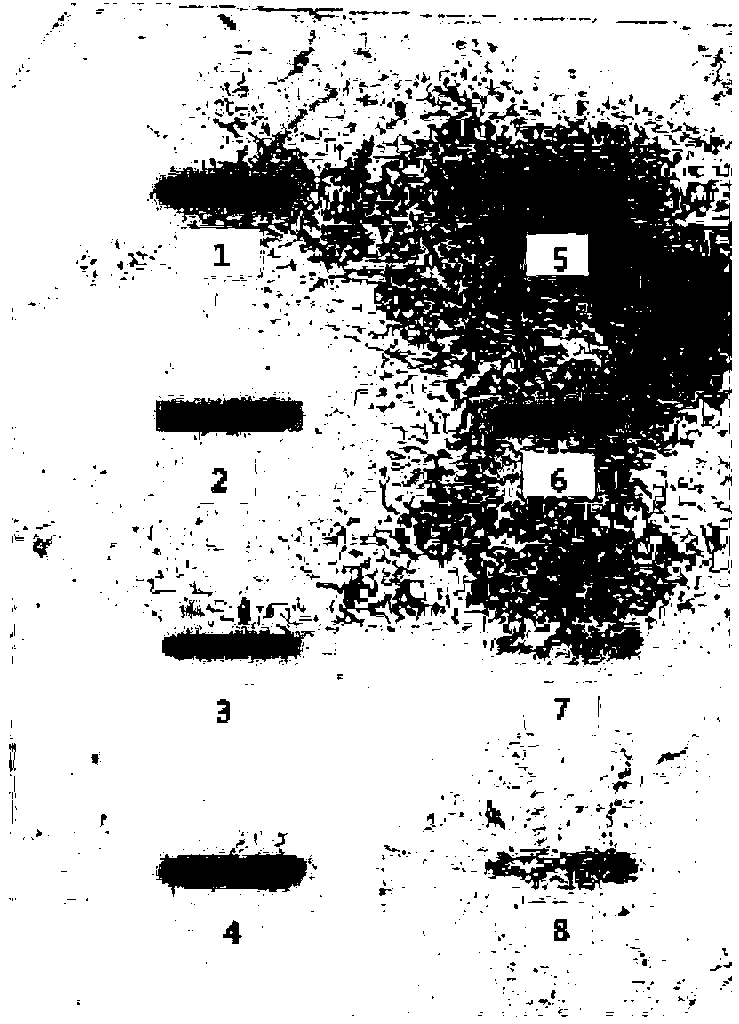
Method for producing HPV31 L1 protein by using Hansenula polymorpha expression system
A Hansenula protein technology, applied in the field of Hansenula expression system to produce HPV31L1 protein, can solve the problems of not providing protein expression level and not disclosing the purity of exogenous protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Analysis of HPV31L1 Consensus Amino Acid Sequence
[0051] The full-length HPV31L1 protein consists of 504 amino acids. After being searched by GenBank, use the AlignX function of the Vector NTI software for amino acid sequence alignment analysis to obtain the most representative HPV31L1 consensus amino acid sequence (consensus amino acid sequence, that is, in each HPV31L1 Amino acid positions all adopt the sequence of amino acid residues with the highest frequency of occurrence), the sequence of which is shown in SEQ ID NO:1.
Embodiment 2
[0052] Example 2: Optimal Design and Artificial Synthesis of HPV31L1 Encoding Gene
[0053] In order to efficiently express the HPV31L1 protein using Hansenula, the inventors optimized the codons of the nucleotide sequence for Hansenula according to the amino acid sequence shown in SEQ ID NO:1. The optimization principles include: a) select codons with the highest or higher frequency of use according to the Hansenula genetic code usage frequency table; b) avoid negative regulatory elements that have potential effects on gene transcription or protein translation, such as PolyAT region, PolyGC region, Silencer region and internal splicing sites, etc.; c) Comprehensive analysis of mRNA secondary structure including 5' end UTR, HPV31L1 coding region and 3' end UTR, to avoid the formation of complex RNA secondary structure, Reduce the free energy of the secondary structure of mRNA; d) Use the 5'UTR region that is completely consistent with the natural sequence downstream of the Han...
Embodiment 3
[0054] According to the above nucleotide sequence, Beijing Nuosai Genome Research Center Co., Ltd. was commissioned to carry out the artificial synthesis of the full sequence, cloned it into a T vector (named T-31hp), and verified it by sequencing. Example 3: Generation of expression constructs carrying the HPV31L1 nucleotide sequence
[0055] The Hansenula expression vector used in the present invention is the Hansenula expression vector pRMHP2.1 (SEQ ID NO: 9) described in the Chinese patent application with application number 201210021524.X.
[0056] (1) PCR amplification of MOX promoter and MOX terminator
[0057] Using the mixed genomic DNA of Hansenula strains ATCC26012 and ATCC34438 as a template, the following primers were used to amplify the MOX promoter with a size of 1518bp, and a NotI restriction site was introduced upstream;
[0058] MOX promoter upstream primer: 5'-AAGGAAAAAAGCGGCCGCAACGATCTCCTCGAGCTGCTCGC-3' (SEQ ID NO: 3)
[0059] MOX promoter downstream prim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com