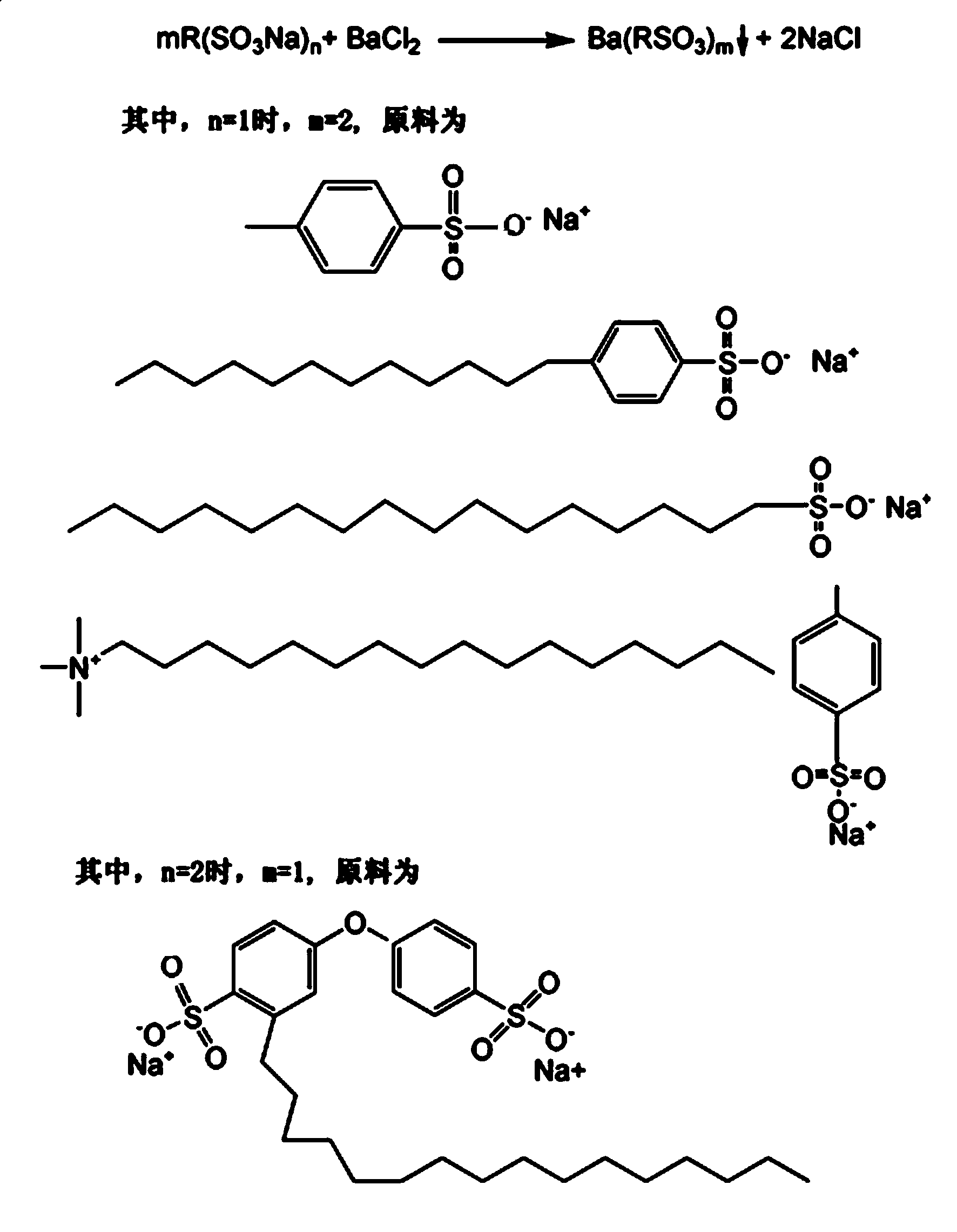
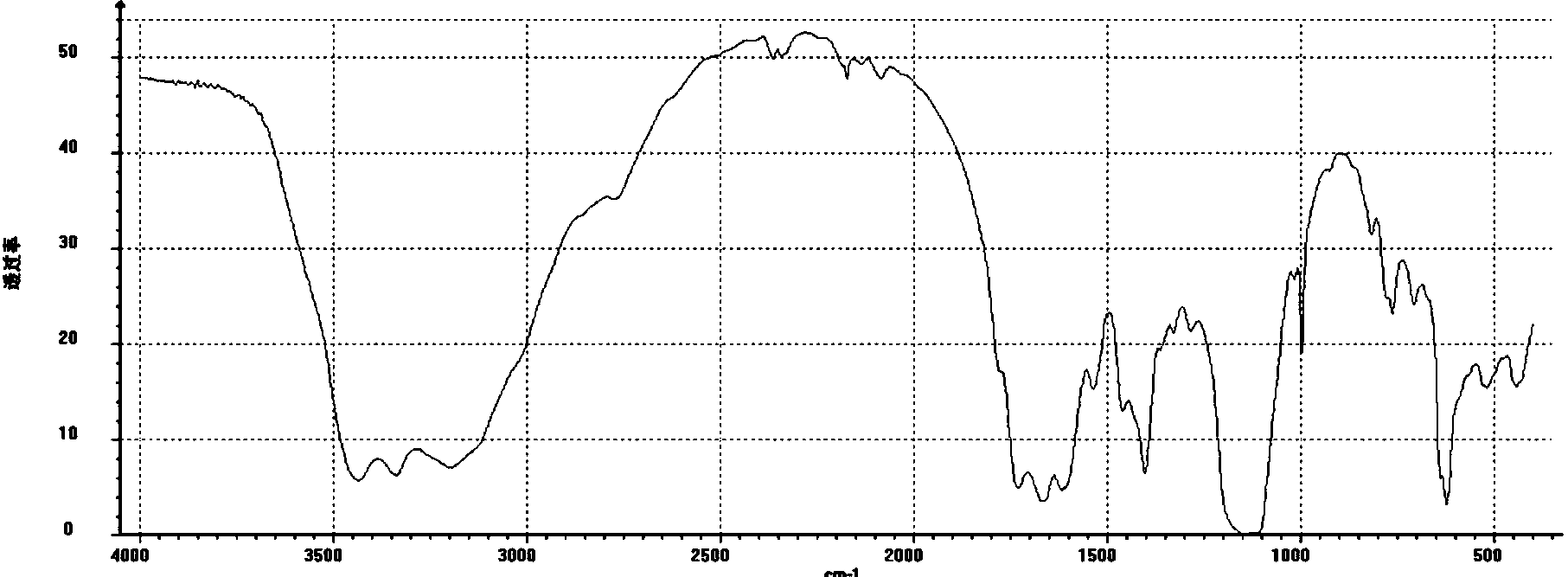
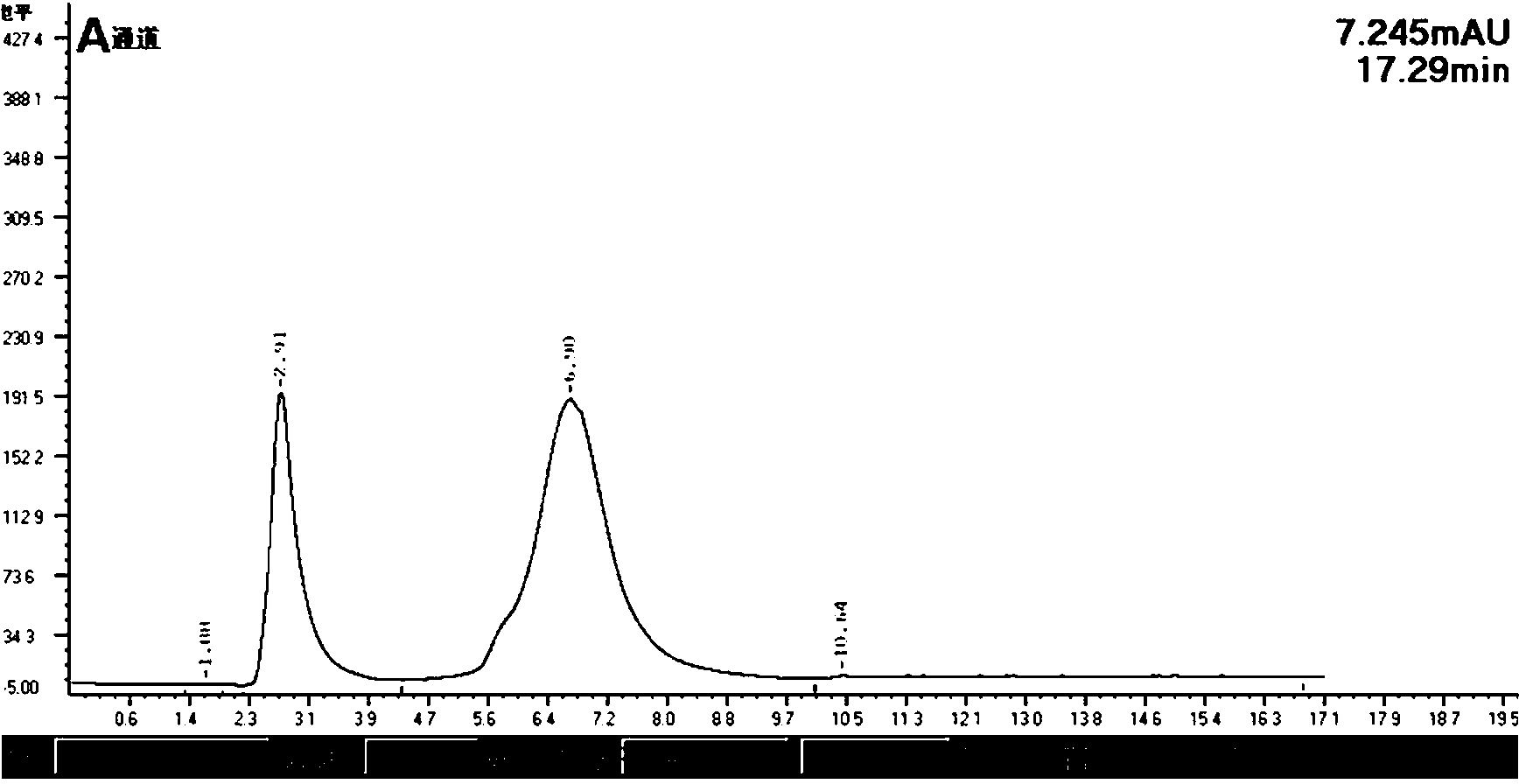
Barium sulfonate catalyst and application thereof in synthesis of D,L-P-hydroxyphenyl hydantoin
A technology of p-hydroxyphenylhydantoin and catalysts, which is applied in the field of catalysts and its applications, can solve the problems of difficult reuse of catalysts and low purity, and achieve the effects of fast synthesis rate, low reaction temperature, and lower reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add sodium p-toluenesulfonate and barium chloride into the ultrasonic reactor, control the initial concentration of sodium p-toluenesulfonate to 0.1 mol / liter, perform ultrasonic reaction at room temperature, control the temperature of the system by circulating condensed water, and react under 400W for 3 hours, and the reaction obtained Put the material into a centrifuge tank, centrifuge to remove the lower layer of sediment, suspend the sediment in water, adjust the apparent concentration to 0.1 mol / liter, stir, age, centrifuge, and dry at low temperature to obtain the catalyst 1 product.
[0033] Chemical reaction formula such as figure 1 As shown, catalyst 1 product yield 89.2%, molecular formula C 14 h 14 o 6 S 2 Ba 1 , white spherical particles, particle size 0.1 ± 0.02 microns, insoluble in water, phenol, elemental analysis: C 35.03%, H 2.92%, O 20.06%.
Embodiment 2
[0035] Add sodium dodecylbenzenesulfonate and barium chloride into an ultrasonic reactor, add water to adjust the initial concentration of sodium dodecylbenzenesulfonate to 0.1 mol / liter, ultrasonically react at 20°C, and control the temperature of the system by circulating condensed water , reacted under 400W ultrasonic conditions for 3 hours, put the reaction product into a centrifuge tank, centrifuge to remove the lower layer of sediment, suspend the sediment in water, adjust the apparent concentration to 0.1 mol / liter, stir and age, then centrifuge, The product of catalyst 2 was obtained by drying at low temperature, and the yield was calculated by weighing.
[0036] Catalyst 2 product yield 86.7%, molecular formula C 36 h 58 o 6 S 2 Ba 1 , white spherical particles, particle size 0.098 ± 0.003 microns, insoluble in water and phenol, elemental analysis: C 54.82%, H 7.48%, O 12.22%.
Embodiment 3
[0038] Put sodium cetyl sulfonate and barium chloride into an ultrasonic reactor, add water to adjust the initial concentration of sodium cetyl sulfonate to 0.1 mol / liter, ultrasonically react, circulate condensed water, control the system temperature, and react for 3 hours Finally, put the reaction product into a centrifuge tank, centrifuge to remove the lower layer of sediment, suspend the sediment in water, adjust the apparent concentration to 0.1 mol / liter, stir and age, centrifuge, and dry at low temperature to obtain the catalyst 3 product, which is weighed and Calculate the yield.
[0039] Catalyst 3 product yield 76.74%, molecular formula C 32 h 66 o 6 S 2 Ba 1, white spherical particles, particle size 0.088 ± 0.013 microns, insoluble in water and phenol, elemental analysis: C 51.16%, H 8.92%, O 12.88%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com