Fluorescence labeling method for pyraoxystrobin and applications thereof
A technology of fluorescent labeling and pyraclostrobin, applied in the directions of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of long half-life and low sensitivity, and achieve the effect of simple steps, high application value and good labeling effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of fluorescently labeled pyraclostrobin with dansyl chloride.
[0024] a. Amidation of pyraclostrobin with ethylenediamine:
[0025] Weigh 412mg (1mmol) of pyraclostrobin, add 10mL of methanol, add 200mg of solid alkali oxide calcined at 500°C, add dropwise 0.67mL (~10mmol) of ethylenediamine, slowly raise the temperature to 40°C and react for 6 hours. After filtration, the reaction solution was evaporated to dryness, and purified by preparative chromatography column chromatography (eluent composed of dichloromethane:methanol=20:1) to obtain 374mg (0.85mmol) pyraclostrobin derivatives, and the yield was 85%; chemical reaction formula as follows:
[0026]
[0027] b. Amidation with dansyl chloride to introduce a fluorescent group:
[0028] Weigh 188mg (0.43mmol) of the above pyraclostrobin derivative, 127mg (~0.47mmol) of dansyl chloride, 5.2mg (0.043mmol) of 4-dimethylaminopyridine (DMAP), then add 2mL of dichloromethane, 72mg (0.71 mmol) triethylamine...
Embodiment 2
[0031] Detection experiment on the relevant properties of the dansyl chloride fluorescently labeled pyraclostrobin prepared in Example 1.
[0032] (1) Determination of the dissociation mechanism of the structural fragment ions of pyraclostrobin.
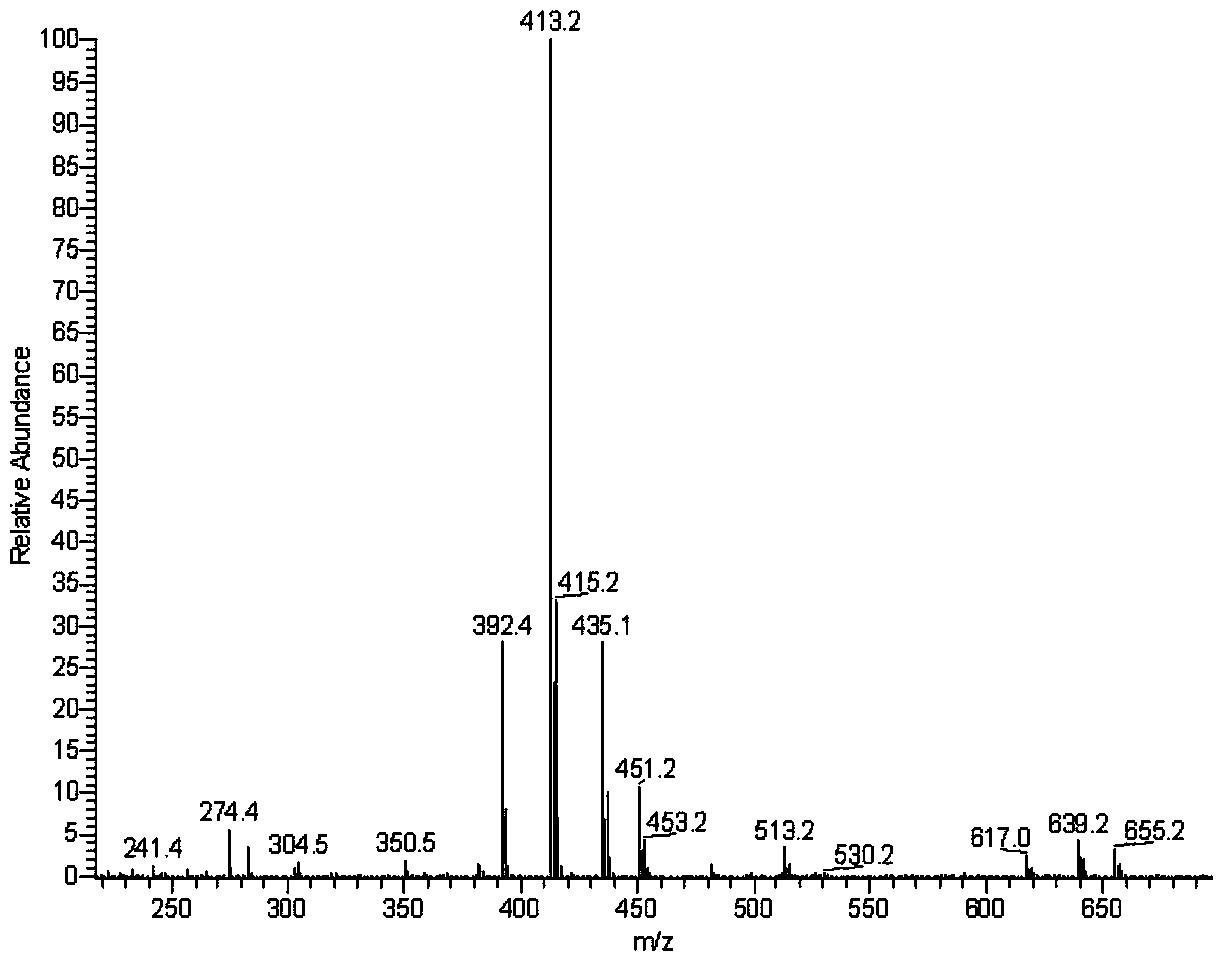
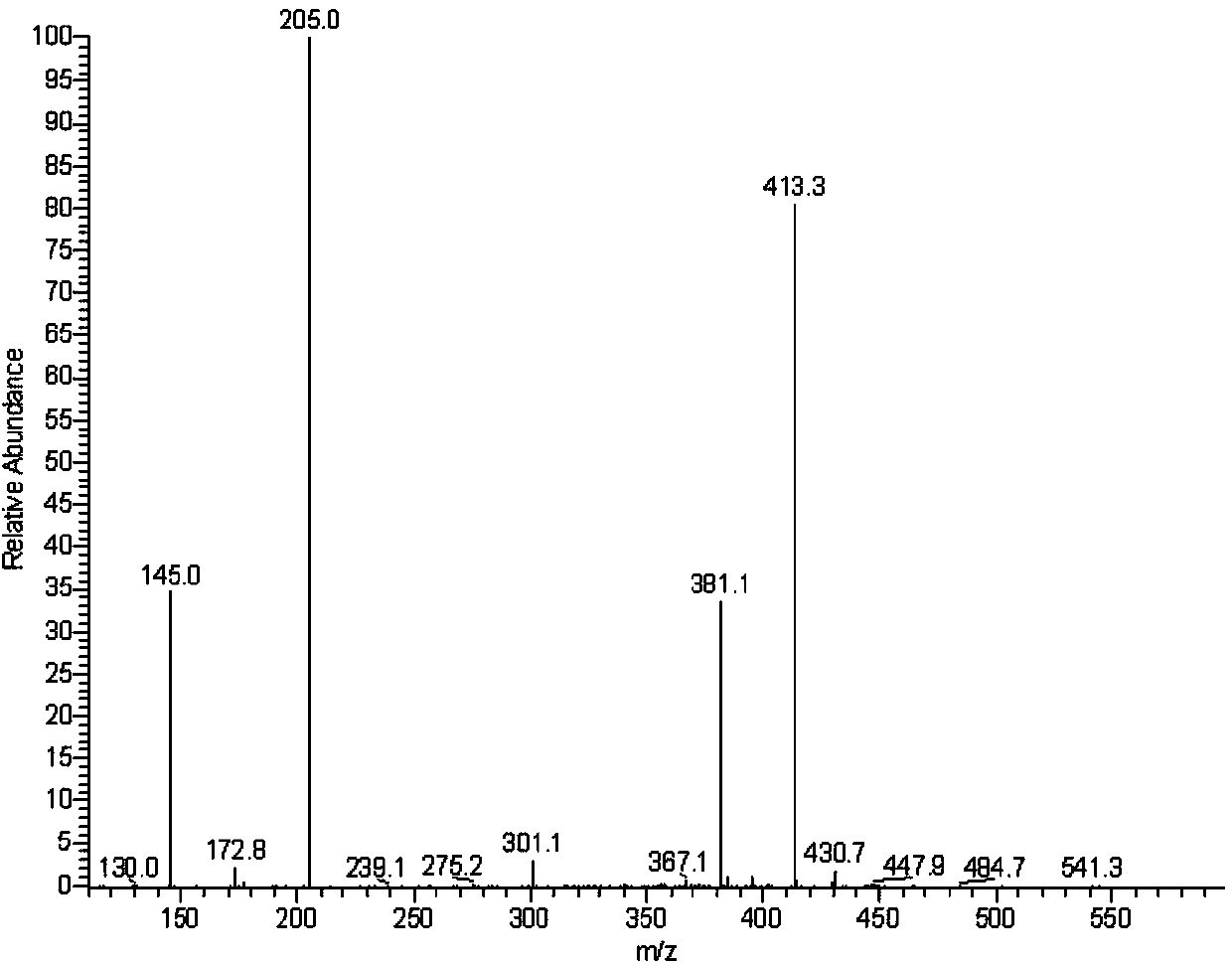
[0033] After the ester group of pyraclostrobin was derivatized with amino reagent, the fluorescent marker dansyl chloride was labeled, and the trace analysis method of pyraclostrobin was established by high performance liquid chromatography, and the metabolism of pyraclostrobin fluorescent marker was designed. experiment method. First through pyraclostrobin full scan mass spectrogram (see figure 1 ), confirm [M+H] + The ion peak is 413.2, 435.1 is [M+Na] + Ion peaks, in [M+H] + Ion peak 413.2 is the precursor ion for secondary mass spectrometry analysis (see figure 2 ). Depend on figure 2 It can be seen that the fragment ions 145.0 and 205.0 with the two strongest signals in the secondary mass spectrum, and the fragment ion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com