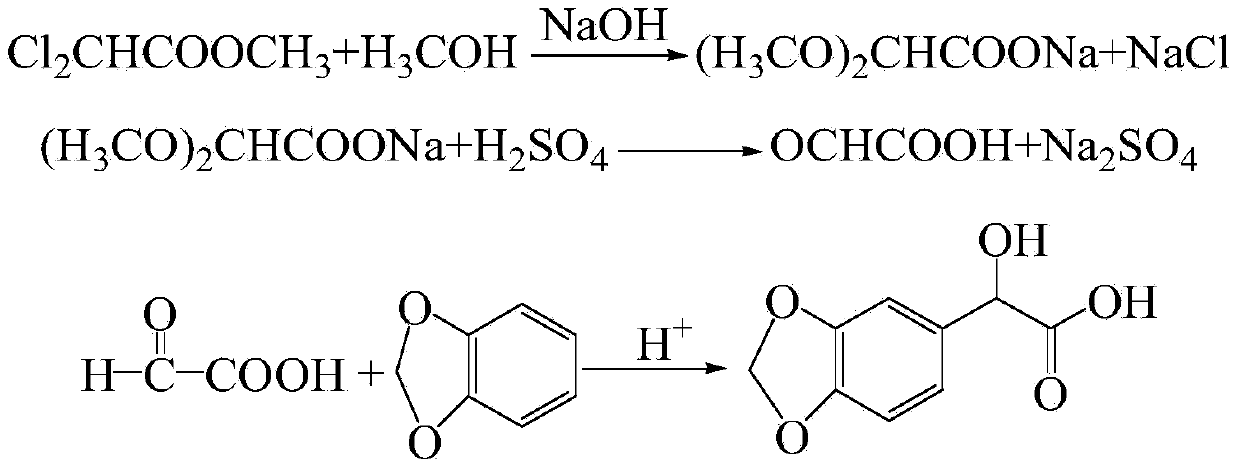
Preparation method for 3,4-methylene dioxy mandelic acid
A technology of methylenedioxybenzene and glycolic acid, which is applied in the direction of organic chemistry, can solve the problems of large equipment investment, high production cost, and equipment corrosion, and achieve low raw material cost, less three wastes, and elimination of energy consumption and three wastes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 45 mL of methanol to a 100-ml three-necked flask, and then add 8.5 g of sodium hydroxide into the methanol and stir to dissolve. After 1 h, 5.2 ml (0.05 mol) of methyl dichloroacetate was added dropwise, and the reaction temperature was controlled at 75°C under continuous microwave radiation heating for 7.5 h to obtain a white opaque solution, and the solid was filtered out. Wash the filter cake with an appropriate amount of methanol, and combine the filtrates. Distill methanol in a constant temperature water bath at 45°C, then add 50% sulfuric acid solution to adjust the pH to 6-8, freeze overnight, filter to desalinate, add 50% sulfuric acid solution to the prepared filtrate to adjust the pH to 2-3, Add 5ml of water and reflux for 3h to obtain 40% glyoxylic acid aqueous solution. The yield is 84.60%.
Embodiment 2-3
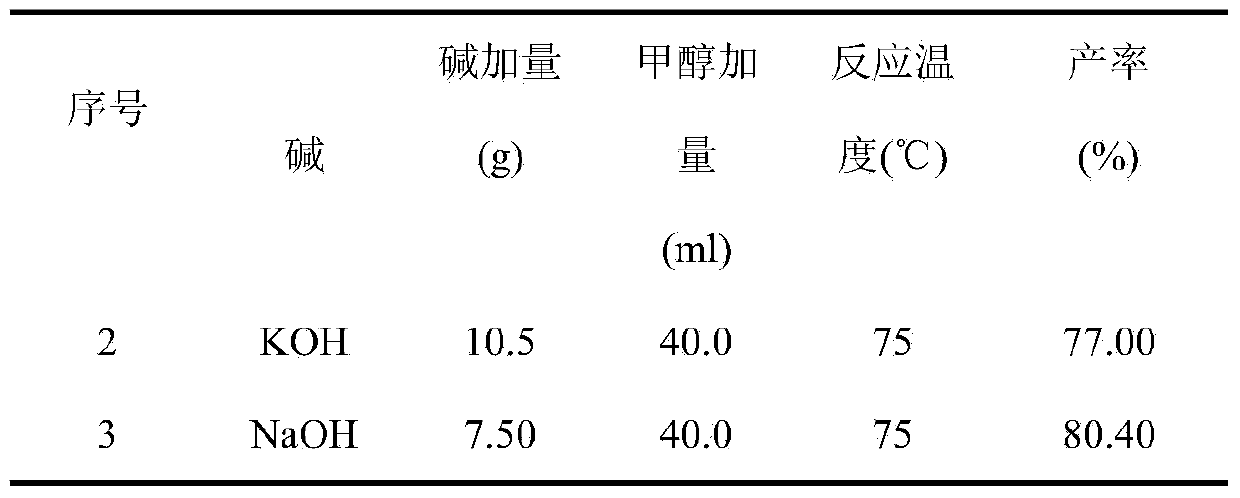
[0024] In Example 2-3, the reaction was carried out in the same manner as in Example 1 except for changing the type of base used, the amount of base added, and the amount of methanol added, and the results are shown in the table below.
[0025]
Embodiment 4-7
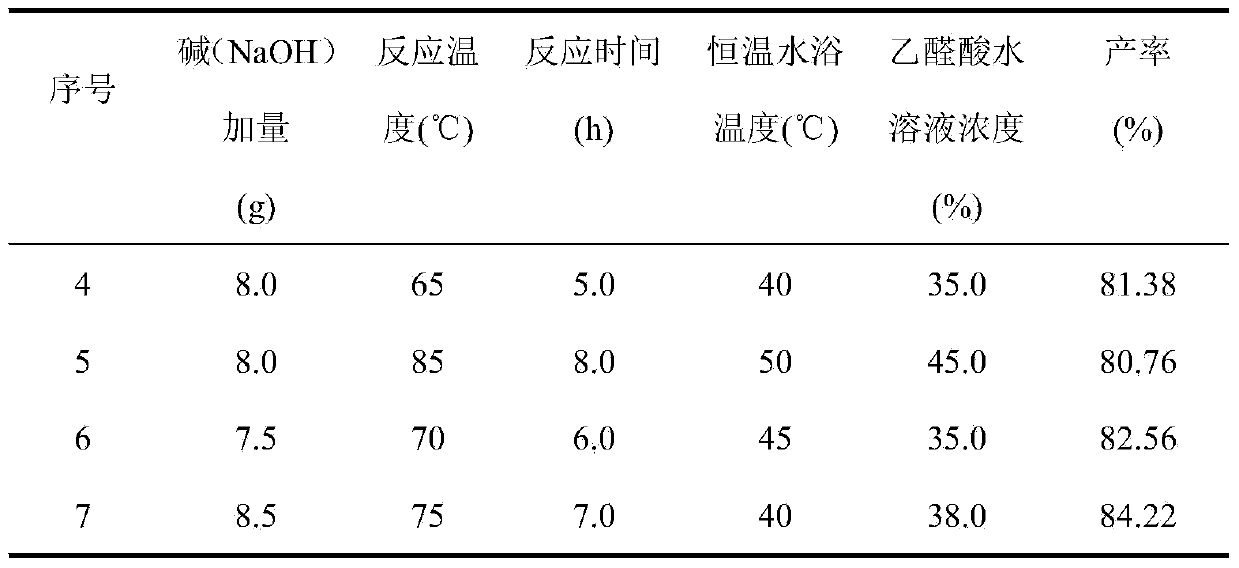
[0027] In Examples 4-7, except changing the amount of alkali (NaOH), reaction temperature and time, and constant temperature water bath temperature, the reaction was carried out in the same manner as in Example 1, and the results are shown in the table below.
[0028]
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com