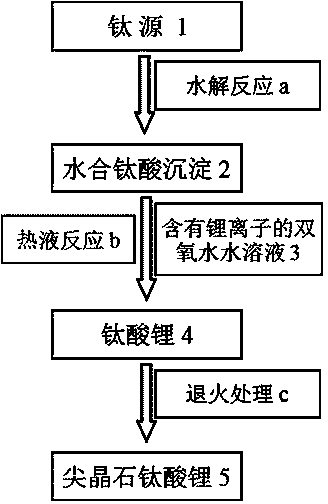
Method for preparing spinel lithium titanate
A spinel type, lithium titanate technology, applied in the directions of titanate, alkali metal titanate, chemical instruments and methods, etc., to achieve low production cost, overcome low tap density, and overcome low electrical conductivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
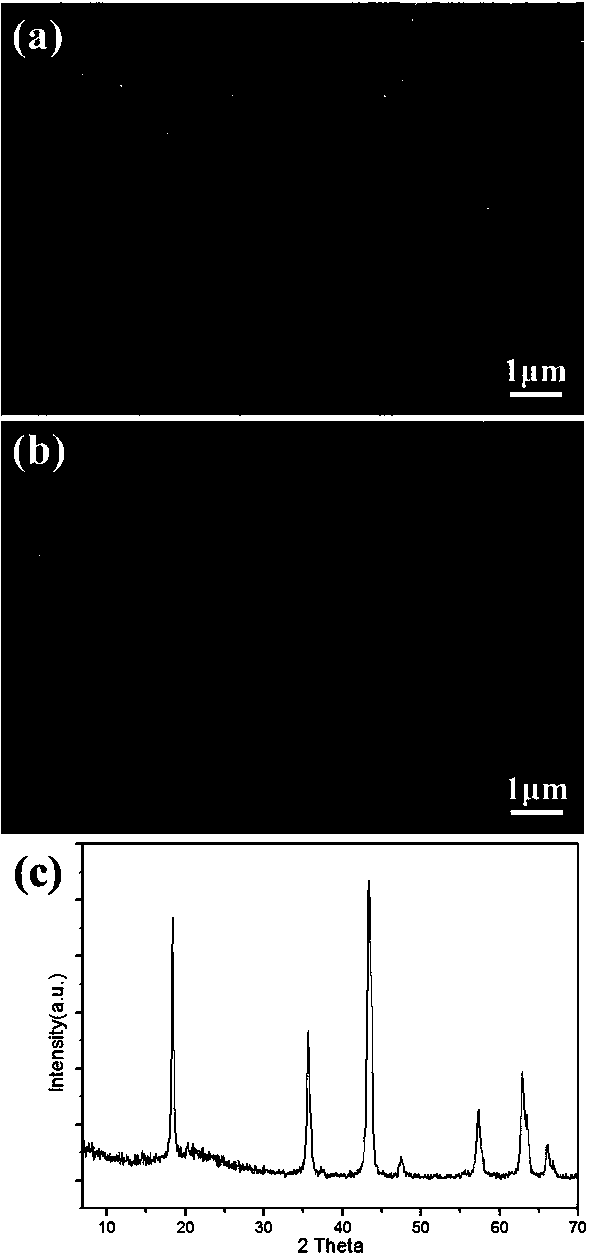
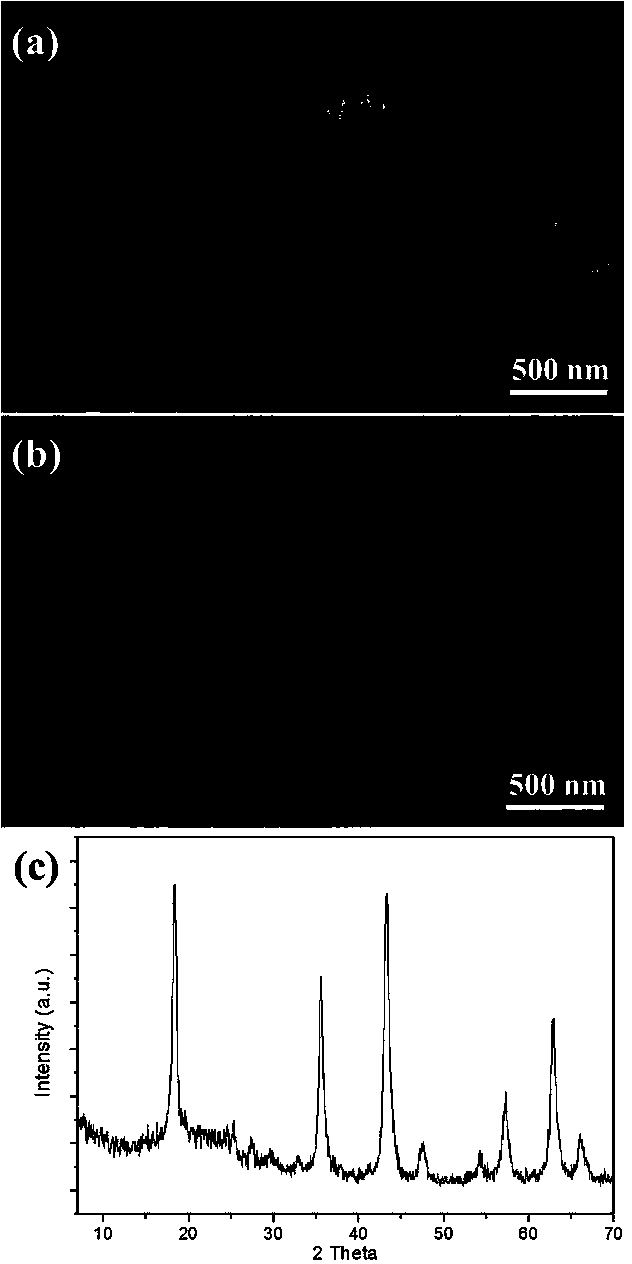
[0051] Under stirring conditions, 0.5 g of titanium sulfate was dispersed and dissolved in 100 ml of deionized water. Ammonia water with a concentration of 0.1 mole per liter is slowly added dropwise to the above solution until the solution is neutral, and the titanium sulfate is gradually hydrolyzed to form hydrous titanic acid precipitation. The hydrated titanic acid precipitate is ultrasonically dispersed, washed with deionized water for many times, and centrifuged to obtain the washed hydrated titanic acid precipitate. Lithium hydroxide was added to a hydrogen peroxide aqueous solution with a volume fraction of 2% hydrogen peroxide to obtain a hydrogen peroxide aqueous solution containing a lithium ion concentration of 0.5 mole per liter. Disperse the washed hydrous titanic acid precipitate in 40 ml of the lithium ion-containing hydrogen peroxide solution to obtain a mixture. After stirring the mixture for 1 hour, hydrothermally react at 100 degrees Celsius for 4 hours, an...
Embodiment 2
[0053] Under stirring conditions, 1 g of titanium sulfate was dispersed and dissolved in 100 ml of deionized water. Sodium hydroxide with a concentration of 0.1 mole per liter is slowly added dropwise to the above solution until the solution is neutral, so that titanium sulfate is gradually hydrolyzed to generate hydrous titanic acid precipitation. The hydrated titanic acid precipitate is ultrasonically dispersed, washed with deionized water for many times, and centrifuged to obtain the washed hydrated titanic acid precipitate. Lithium hydroxide was added to a hydrogen peroxide aqueous solution with a volume fraction of 2% hydrogen peroxide to obtain a hydrogen peroxide aqueous solution containing a lithium ion concentration of 0.2 mole per liter. Disperse the washed hydrated titanic acid precipitate in 80 ml of the hydrogen peroxide aqueous solution containing lithium ions to obtain a mixture. After stirring the mixture for 2 hours, it was subjected to hydrothermal reaction a...
Embodiment 3
[0055] Under stirring conditions, 1 g of titanium sulfate was dispersed and dissolved in 100 ml of deionized water. Sodium hydroxide with a concentration of 0.1 mole per liter is slowly added dropwise to the above solution until the solution is neutral, so that titanium sulfate is gradually hydrolyzed to generate hydrous titanic acid precipitation. The hydrated titanic acid precipitate is ultrasonically dispersed, washed with deionized water for many times, and centrifuged to obtain the washed hydrated titanic acid precipitate. Lithium hydroxide was added to a hydrogen peroxide solution with a volume fraction of 2% to obtain a hydrogen peroxide solution containing a lithium ion concentration of 1 mole per liter. Disperse the washed hydrated titanic acid precipitate in 80 ml of the hydrogen peroxide aqueous solution containing lithium ions to obtain a mixture. After stirring the mixture for 1 hour, it was subjected to hydrothermal reaction at 100 degrees Celsius for 4 hours, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com