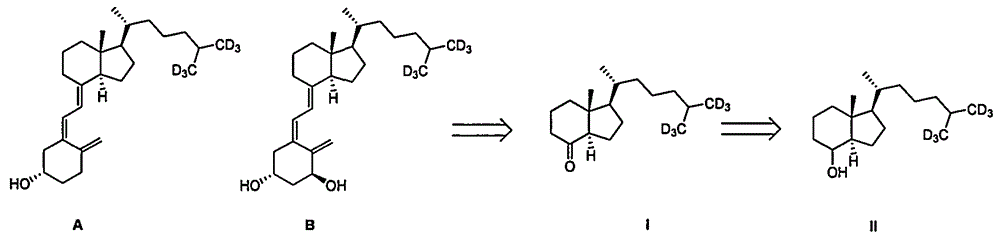
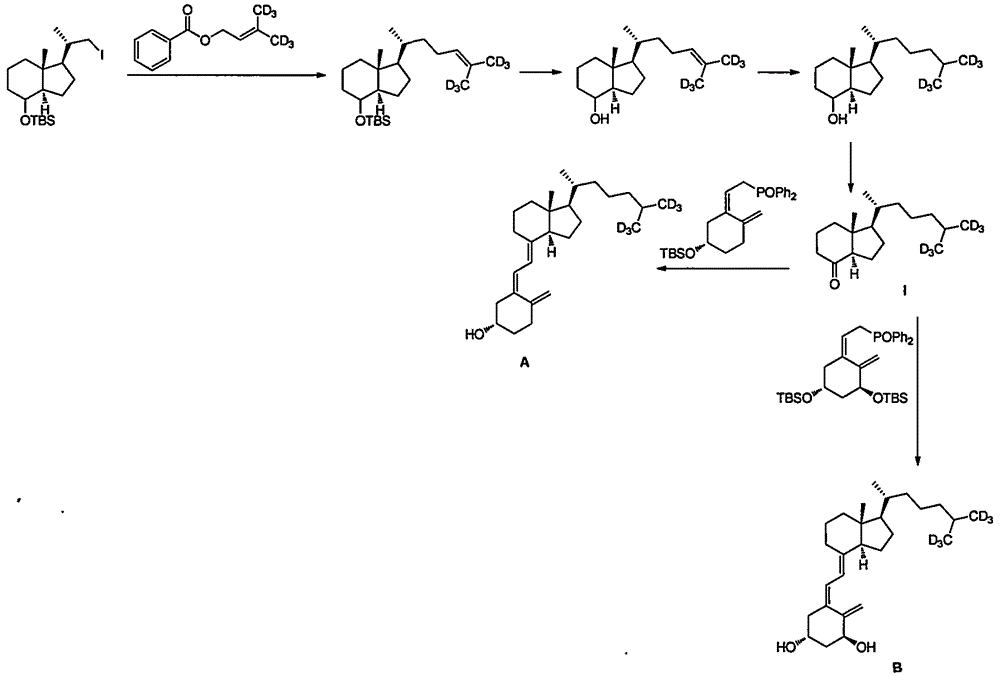
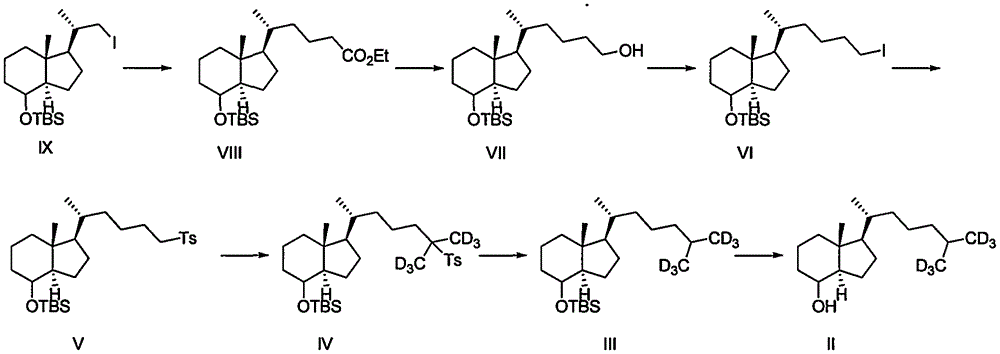
A method for preparing intermediate compound ii of vitamin D and its analogs
A technology of compounds and analogues, which is applied in the field of compound preparation, can solve the problems of non-commercialization of prenol benzoate raw materials, difficult costs, and easy danger, so as to reduce the risk of preparation, easy to scale up preparation, and reduce cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of compound VIII
[0037]
[0038] Pyridine (300mL), NiCl 2.6H 2 O (21g, 73.31mmol) and ethyl acrylate (36.65g, 320mmol, 4.4eq) were added into a 1L three-necked flask, and the temperature was raised to 60°C. After the reaction solution turned reddish brown, the temperature was kept constant and stirred for 4 hours, and then The reaction solution was cooled to room temperature, and 20 mL of a pyridine solution of IX (32 g, 73.31 mmol) was added dropwise, and the temperature was kept stirring after dropping.
[0039] 40 minutes after the raw material was added dropwise, TLC detected that the raw material spot disappeared, indicating that the reaction was complete. Quench the reaction with 1mol / L hydrochloric acid solution (1L), extract with ethyl acetate, combine the organic phases, wash with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate to obtain 31g brown oily liquid crude product. Silica gel column chromatography gave 2...
Embodiment 2
[0041] Embodiment 2: the preparation of compound VII
[0042]
[0043] Dissolve VIII (22g, 53.5mmol) in THF (250mL), cool down to 10°C in an ice bath, and add LiAlH in batches 4 (3g, 80.3mmol), after the addition, the ice room temperature reaction was removed. After 40 minutes, TLC observed that the raw material spots disappeared, and the reaction was completed, and the aqueous solution of 1mol / LNaOH was added dropwise to the excess LiAlH 4 Just quenched. The reaction solution was filtered through diatomaceous earth, the filter cake was washed with ethyl acetate, and the filtrate was concentrated to obtain 20 g of crude yellow oil. Silica gel column chromatography gave 18 g of colorless oil VII, with a yield of 91%.
[0044] 1H-NMR (CDCl3, δ): 3.98 (1H, brs), 3.63 (2H, t, J=6.85Hz), 1.93 (1H, m), 1.87-1.75 (3H, m), 1.58-1.40 (5H, m), 1.35-1.28 (7H, m), 1.40-1.09 (2H, m), 0.97 (3H, d, J=6.8), 0.94 (3H, s), 0.89 (9H, s), 0.025 (6H, s); HRMS(ESI-TOF): calcdfor[C 22 h 44...
Embodiment 3
[0045] Embodiment 3: the preparation of compound VI
[0046]
[0047] Dissolve VII (18g, 48.82mmol) in 360mLTHF, add imidazole (10.5g, 146.46mmol) and triphenylphosphine (25.6g, 97.64mmol), cool down to 5°C, add iodine (24.8g, 97.64mmol) dropwise 10 mL of THF solution. After 30 minutes, TLC observed that the raw material spots disappeared, added 1L of water to quench, extracted with ethyl acetate, combined organic phases, washed with saturated NaCl, anhydrous NaCl 2 SO 4 Drying and concentration gave 40 g of crude yellow oil. Silica gel column chromatography gave 21 g of light yellow oil VI, with a yield of 90%.
[0048] 1HNMR (400MHz, CDCI3) δ3.99(1H, s), 2.60(2H, t, J=7.46Hz), 1.90(1H, d, J=12.8Hz), 1.84-1.76(2H, m), 1.66( 1H, d, J=13.6Hz), 1.59-1.41(5H, m), 1.40-1.08(8H, m), 0.98(3H, d, J=5.2Hz), 0.94(3H, s), 0.88(9H , s), -0.00(3H, s), -0.02(3H, s); HRMS (ESI-TOF): calcdfor[C 22 h 43 IOSi+H]+478.21, found 478.21.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com