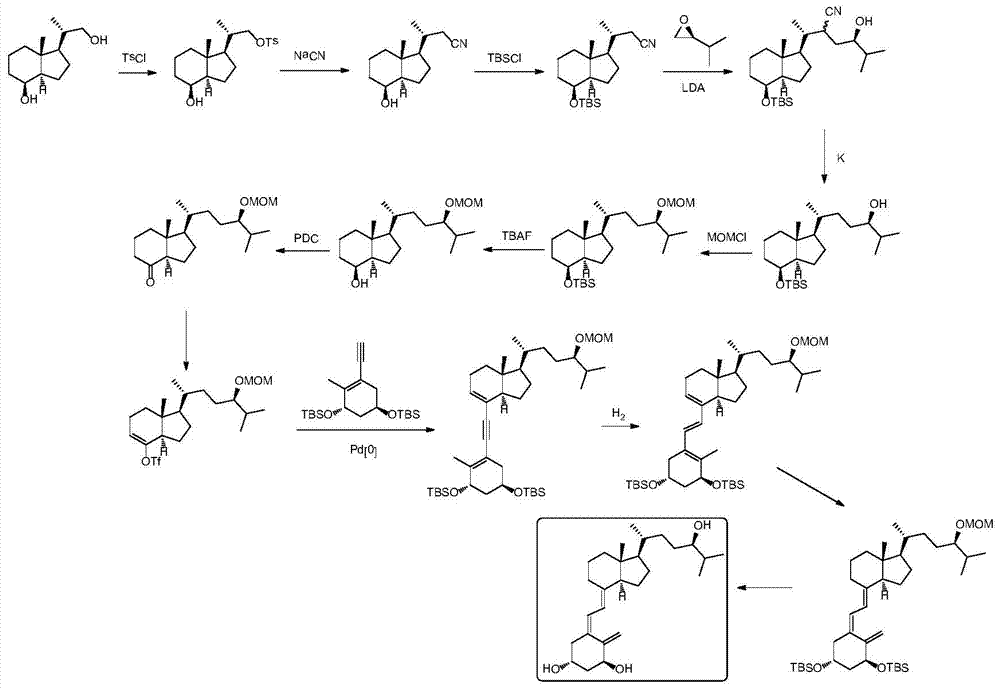
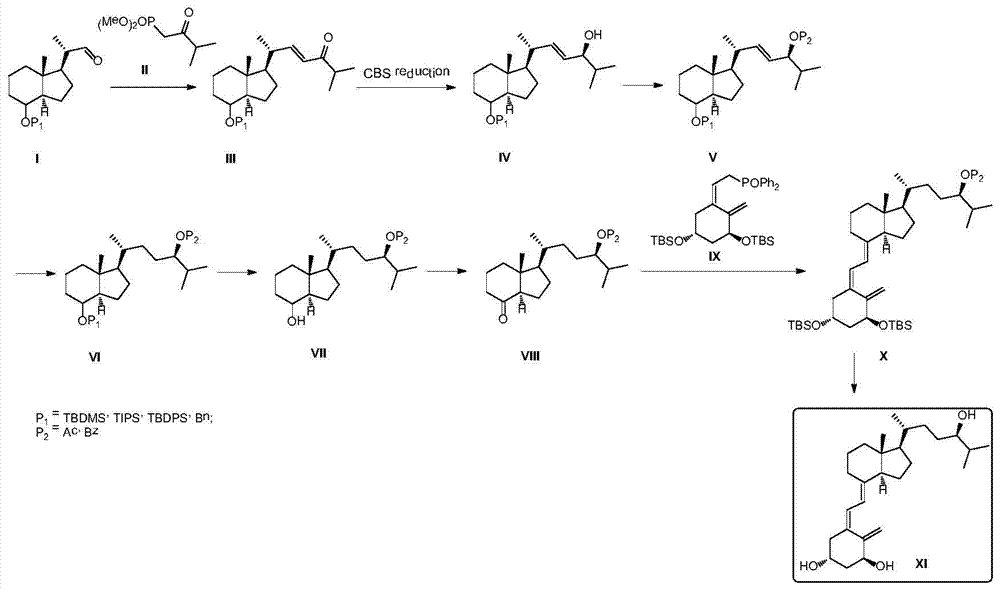
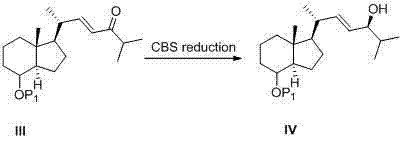
Method for preparing tacalcitol
A technology of tacalcitol and compounds, which is applied in the field of compound preparation, can solve the problems of long route, unfavorable industrial production, and easy occurrence of dangerous environmental pollution, etc., achieves high stereoselectivity, easy scale-up preparation, and reduces the effect of preparation risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of compound IIIa
[0047]
[0048] Compound II (13 g, 66.6 mmol) and anhydrous lithium chloride (3.85 g, 88.8 mmol) were dissolved in 300 mL of acetonitrile, triethylamine (10.2 g, 88.8 mmol) was added dropwise under nitrogen protection, and the temperature was raised to 50 degrees And keep the temperature for reaction for 1 hour, add compound Ia (14.4 g, 44.4 mmol), keep stirring at 50°C for 10 hours, TLC detects that the raw material spots disappear, and the reaction is complete. Quench the reaction with 200 mL of saturated ammonium chloride solution, extract twice with 200 mL of n-hexane, combine the organic phases, wash with 200 mL of saturated brine, wash with 200 g of anhydrous Na 2 SO 4 Drying and concentration under pressure gave 26 g of the crude product, which was chromatographed on a silica gel column to give 14.6 g of Compound III with a yield of 87%. 95% purity.
[0049] 1H NMR (400MHz, CDCl 3 ) δ=6.75 (1H, dd, J=15.4, 5...
Embodiment 2
[0050] Embodiment 2: the preparation of compound IIIb
[0051]
[0052] Acetonitrile (400 mL), lithium chloride (7.53 g, 177.6 mmol) and compound II (26 g, 133.2 mmol) were added to a dry 1 L three-necked flask, and triethylamine (22.4 g, 221.8 mmol), heated to 50°C and the reaction solution was dissolved until clear, kept stirring at 50°C for 1 h, added compound Ib (14.4 g, 44.36 mmol) in dichloromethane solution, kept stirring at 50°C for 13 hours, TLC detected that the raw material spots disappeared , quenched by adding 500 mL of saturated NH4Cl aqueous solution, extracted three times with 300 mL of n-hexane, combined the organic phases, washed with 200 mL of saturated NaCl aqueous solution, dried with 200 g of anhydrous Na2SO4, concentrated under reduced pressure to obtain 20 g of crude yellow oil, passed through a silica gel column layer 13.8 g of yellow oil was separated out, the yield was 79.3%, and the purity was 95%.
[0053] 1H NMR (400MHz, CDCl 3 ) δ=6.75 (1H, ...
Embodiment 3
[0054] Embodiment 3: the preparation of compound IIIc
[0055]
[0056] Add acetonitrile (300 mL), lithium chloride (10.5 g, 250 mmol) and compound II (4838 g, 250 mmol) into a dry 1 L three-necked flask, and add triethylamine (38 g, 250 mmol ), heated to 50°C and the reaction solution was dissolved until clarified, kept stirring at 50°C for 1 h, added a dichloromethane solution of compound Id (20 g, 50 mmol), kept stirring at 50°C for 13 hours, TLC detected that the raw material spots disappeared, Quenched by adding 500 mL of saturated NH4Cl aqueous solution, extracted three times with 300 mL of n-hexane, combined the organic phases, washed with 200 mL of saturated NaCl aqueous solution, dried with 200 g of anhydrous Na2SO4, concentrated under reduced pressure to obtain 16 g of crude yellow oil, passed through a silica gel column layer 11.4 g of compound IIIc was obtained with a yield of 89% and a purity of 98%.
[0057] 1H NMR (400MHz, CDCl 3 ) δ=6.75 (1H, dd, J=15.4, 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com