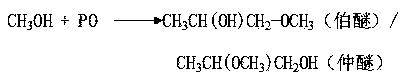Method for preparing dipropylene glycol methyl ether
A technology of dipropylene glycol methyl ether and propylene glycol methyl ether, which is applied in the field of preparing dipropylene glycol methyl ether, can solve the problems of energy consumption and low market value of 2-methoxy-1-propanol, and achieves the effect of saving energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A method for preparing dipropylene glycol methyl ether, the specific steps are as follows:
[0049] (1) The reaction between the tower kettle liquid after the extraction of propylene glycol methyl ether and propylene oxide is specifically as follows:
[0050] (1a) The mass fraction of water in a certain batch of tower liquid used as raw materials measured by Karl Fischer moisture meter is 0.05%; the mass fraction of sodium methoxide in the tower liquid measured by the volumetric method of acid-base titration is 2.68 %; Analyze on a gas chromatograph with a 25m×0.32mm×0.5μm FFAP strongly polar capillary column, and multiply the area percentage of each component by (1-mass fraction of water-mass fraction of sodium methoxide), The mass fractions of some components that can be detected by gas chromatography in this batch of tower still liquid are:
[0051] Propylene glycol methyl ether 0.15%
[0052] 2-methoxy-1-propanol 77.67%
[0053] Dipropylene glycol methyl ether 17.45%
[0054...
Embodiment 2
[0088] On the basis of Example 1, other conditions remain unchanged, except that the mass fraction of the flaky sodium hydroxide used in steps (1a), (1b) and (1c) of Example 1 is changed to 97.2% of the granular solid sodium methoxide, and the mass of sodium methoxide added in step (1a) is still 2.3g, but the moles of sodium methoxide added are the total moles of propylene glycol methyl ether and 2-methoxy-1-propanol 0.010 times the number of 4 mol, and finally 1900 mL of dipropylene glycol methyl ether is obtained.
[0089] Verification test: Using FFAP strong polarity capillary column, the total mass fraction of the four isomers of dipropylene glycol methyl ether measured on the gas chromatograph is 97.7%.
[0090] Take a 100 mL sample of dipropylene glycol methyl ether and use a special device for measuring boiling range to determine that the boiling range of dipropylene glycol methyl ether is 182.6~194.4°C.
[0091] The boiling range of the dipropylene glycol methyl ether prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com