A modification method of lithium-ion battery cathode lithium-rich material
A lithium-ion battery and lithium-rich material technology, which is applied in the field of preparation and modification of lithium-ion battery cathode materials, can solve the problems of affecting the discharge specific capacity of materials, the improvement of electrochemical performance is not obvious, and the cycle performance is improved, so as to achieve high discharge capacity and Coulombic efficiency, good cycle capacity retention and rate characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
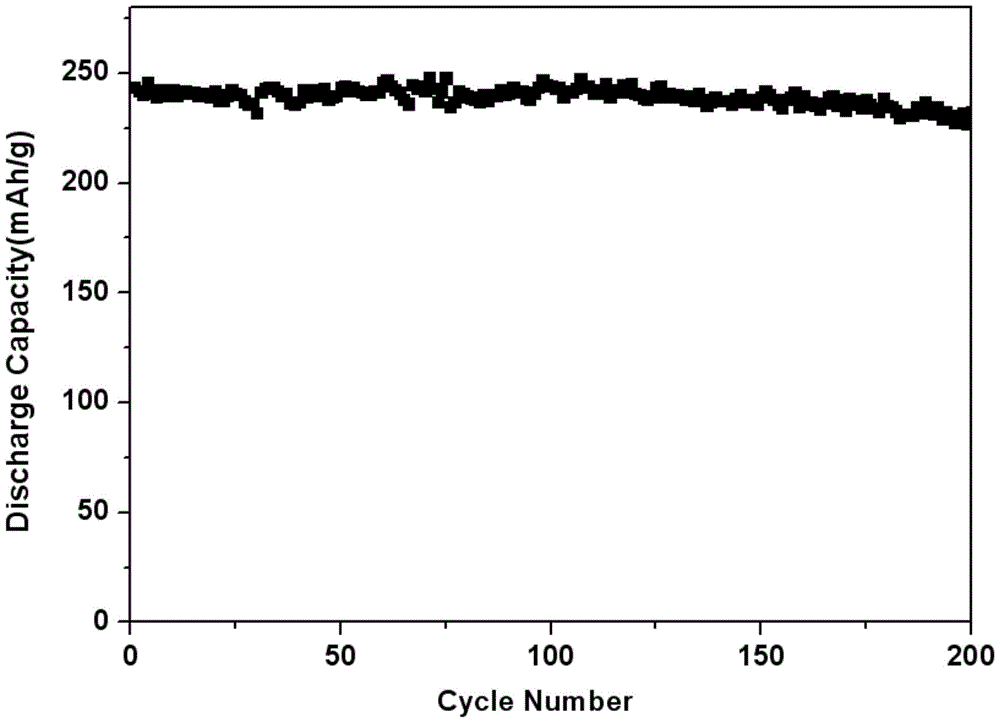
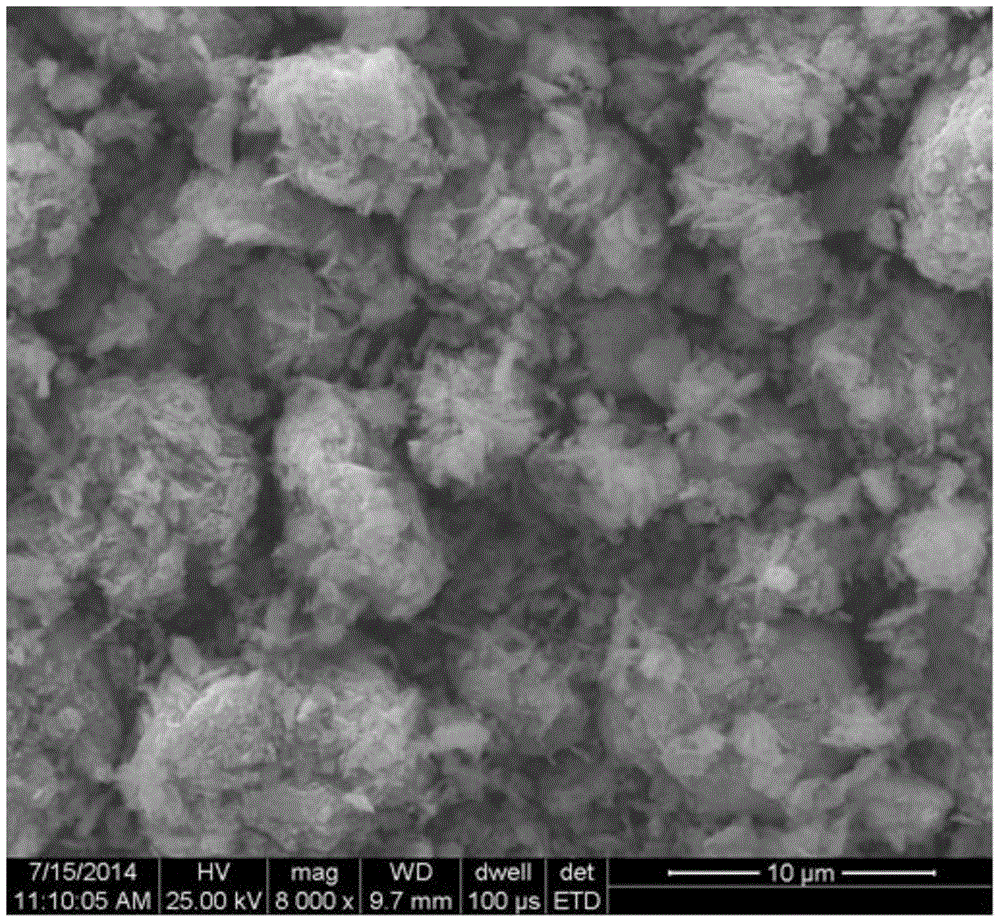
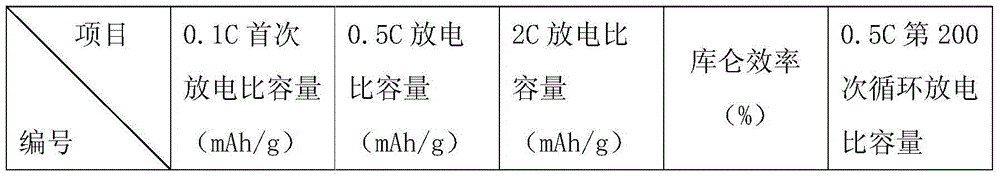
Image
Examples
Embodiment 1
[0066] The specific implementation steps are:
[0067] (1) Add nickel nitrate, manganese nitrate and chromium nitrate to the aqueous solution according to the molar ratio of 0.246:0.746:0.008 to prepare a mixed solution. After stirring for a period of time, add 2M Na 2 CO 3 Solution, the nickel ions, manganese ions and chromium ions in the solution are completely precipitated, suction filtered, washed, and dried at 100°C for 12 hours to obtain a ternary compound containing Ni, Mn, Cr;
[0068] (2) ultrasonically disperse the ternary complex obtained in step (1) in absolute ethanol. According to the mass fraction of the final coated lithium titanate being 5%, wherein the molar weight of titanium remains constant, a certain amount of tetrabutyl titanate is dissolved in absolute ethanol, and slowly added to the above precursor suspension , stirred for 5h. Add 30ml of water / ethanol solution with a volume ratio of 1:2 to the above solution, continue stirring for 5 hours, centrif...
Embodiment 2
[0073] The specific implementation steps are:
[0074] (1) Add nickel acetate, manganese acetate and cobalt acetate into the aqueous solution according to the molar ratio of 0.242:0.742:0.016 to prepare a mixed solution. After stirring for a period of time, add 2M K 2 CO 3 Solution, the nickel ions, manganese ions and chromium ions in the solution are completely precipitated, suction filtered, washed, and dried at 100°C for 12 hours to obtain a ternary compound containing Ni, Mn, Co;
[0075] (2) ultrasonically disperse the ternary complex obtained in step (1) in absolute ethanol. According to the mass fraction of the final coated lithium titanate being 10%, wherein the molar weight of titanium remains constant, a certain amount of tetraisopropyl titanate is dissolved in absolute ethanol, and slowly added to the above precursor suspension In, stir for 3h. Add 30ml of water / ethanol solution with a volume ratio of water and ethanol of 1:4 to the above solution, continue to st...
Embodiment 3
[0078] The specific implementation steps are:
[0079] (1) Add nickel sulfate, manganese sulfate and cobalt sulfate into the aqueous solution according to the molar ratio of 0.238:0.738:0.024 to prepare a mixed solution. After stirring for a period of time, add 2M KOH solution to make the nickel ion, manganese ion and Cobalt ions were completely precipitated, filtered, washed, and dried at 100°C for 12 hours to obtain a ternary compound containing Ni, Mn, and Co;
[0080] (2) ultrasonically disperse the ternary complex obtained in step (1) in absolute ethanol. According to the mass fraction of the final coated lithium titanate being 15%, wherein the molar weight of titanium is always constant, a certain amount of titanium tetrachloride is dissolved in absolute ethanol, and slowly added to the above-mentioned precursor suspension, Stir for 4h. Add 30ml of water / ethanol solution with a volume ratio of 1:6 to the above solution, continue to stir for 4 hours, centrifuge, and was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com