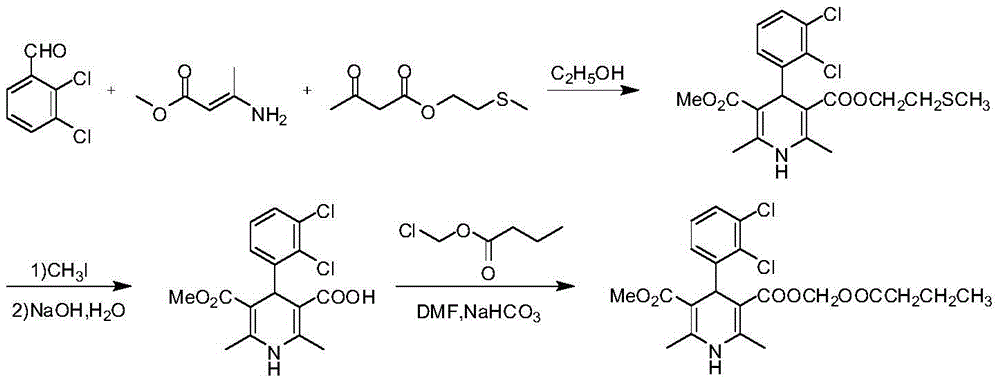
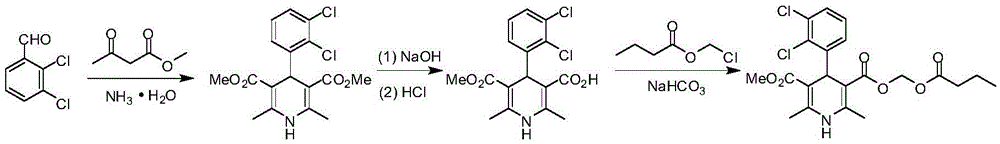
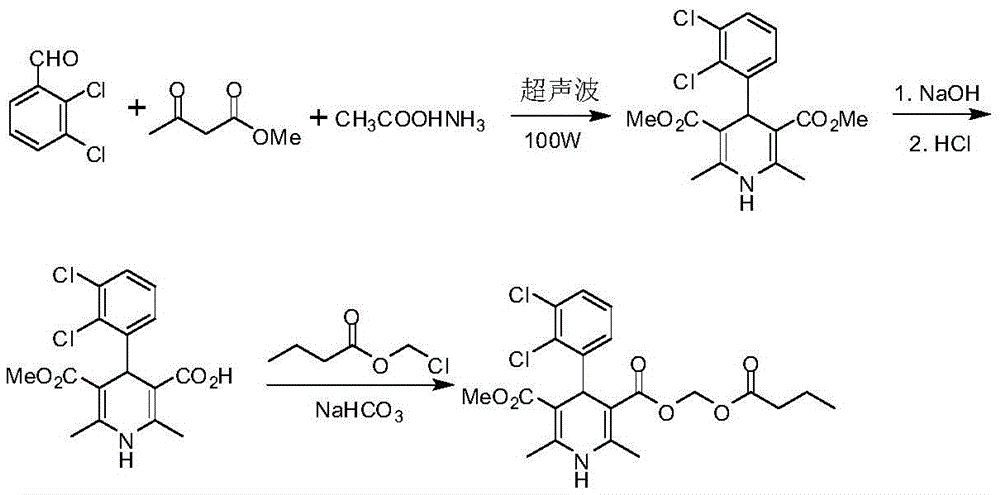
The synthetic method of clevidipine butyrate
A technology of clevidipine butyrate and a synthetic method, which is applied in the chemical field, can solve problems such as inability to carry out industrial scale-up reactions, enzymatic hydrolysis reactions are not suitable for industrial production, and limit the size of reaction vessels, so as to achieve large-scale industrial production and low cost , the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (a) Synthesis of Compound 3
[0038]
[0039] Compound 1 (2g, 13.4mmol) was dissolved in tetrahydrofuran (15ml), triethylamine (176mg) was added, compound 2 (2.1g, 24.8mmol) was added dropwise to the solution, and the dropwise addition was completed, heated to reflux at 60°C, and stirred overnight . Stop the reaction, add saturated citric acid (20ml) after the reaction solution is cooled, extract with dichloromethane, wash the organic layer with water, wash with saturated brine, and wash with anhydrous NaSO 4 After drying, compound 3 (2.2 g, 71% yield) was obtained by separation by column chromatography.
[0040] conduct 1 H-NMR nuclear magnetic resonance analysis result is as follows:
[0041] 1 H-NMR (CDCl 3 ,400MHz,δppm):4.81(s,2H,-CH 2 ),3.61(s,2H,-CH 2 ),2.28(s,3H,-CH 3 ).
[0042] (b) Synthesis of Compound 6
[0043]
[0044] Compound 4 (1.1g, 6.4mmol), Compound 3 (1.5g, 6.4mmol), Compound 5 (0.738g, 6.4mmol) were dissolved in ethanol (15ml), heate...
Embodiment 2
[0058]
[0059] Compound 6 (1g, 2.05mmol) was dissolved in tetrahydrofuran (10ml), under nitrogen protection, SmI was added 2 (5.8g, 14.3mmol), stirred at room temperature for 2h. The reaction solution was concentrated and evaporated to remove the solvent, added DMF to dissolve, filtered to remove insoluble matter, evaporated under reduced pressure to remove most of the DMF, added water, a large amount of solids were precipitated, filtered with suction, and the filter cake was rinsed with an eluent of PE:EA=20:1, Compound 7 (0.32 g, 44% yield) was obtained after oven drying.
Embodiment 3
[0061]
[0062] Compound 6 (1g, 2.05mmol) was dissolved in toluene (11ml), sodium (0.47g, 20.5mmol) was added, and stirred overnight at room temperature. The reaction solution was filtered, concentrated and evaporated to remove the solvent, added water, a large amount of solids were precipitated, filtered with suction, the filter cake was rinsed with PE:EA=20:1 eluent, and dried in an oven to obtain compound 7 (0.27g, 37% yield ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com