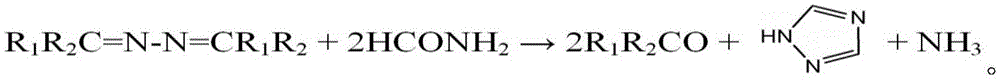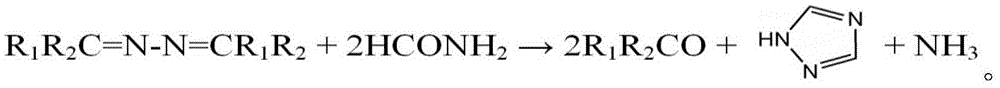The synthetic method of triazole
A synthetic method, triazole technology, applied in the field of organic chemical synthesis, can solve the problems of no obvious advantages in comprehensive cost, difficult separation of formamide, high production cost, etc., and achieve low comprehensive cost, low equipment requirements, and few by-products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
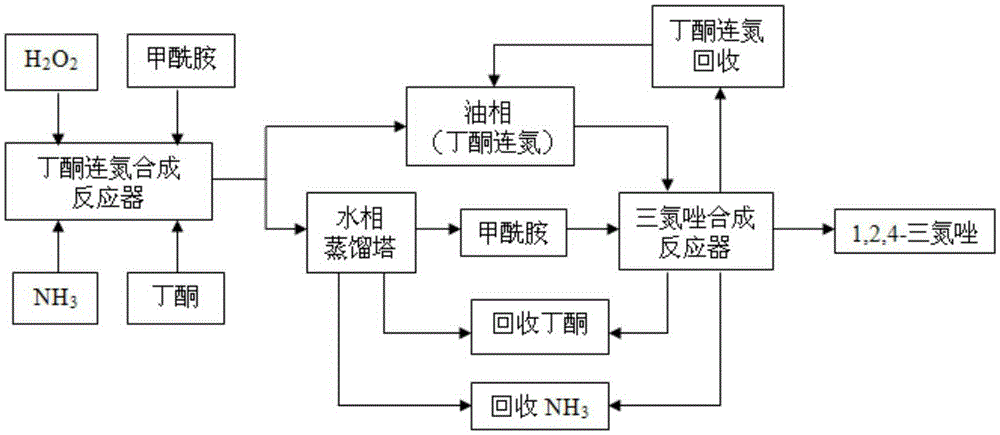
[0034] Technical scheme of the present invention is the synthetic method of triazole, comprises the steps:
[0035] The first step: reacting ammonia, hydrogen peroxide and butanone in the presence of formamide to prepare butanazine;
[0036] Second step: separating the upper stratum oil phase containing butanazine from the aqueous phase containing formamide;
[0037] The third step: distill the ammonia and butanone in the water phase back to the first step, and distill all the water in the water phase to recover formamide;
[0038] The fourth step: raise the temperature of formamide to 165-190°C, then drop the oil phase into the formamide, and the ammonia and methyl ethyl ketone generated by the reaction are reused in the first step; The temperature is kept at ℃ for 20-60 minutes, and the unreacted butanone azine is distilled and recovered to obtain 1,2,4-triazole.
[0039] Preferably, in the fourth step, the temperature of formamide is raised to 170-175° C., and then the oi...
Embodiment 1
[0058] The reaction process is as figure 1 shown. 216g methyl ethyl ketone, 67.5g formamide (1.5mol) and 340g ammoniacal liquor (mass fraction is 20%) join in the four-neck flask that has reflux condenser, thermometer, dropping funnel and stirring, add mass in the dropping funnel The fraction is 113.3g (1mol) of 30% hydrogen peroxide, heat the reaction solution to 45°C, add hydrogen peroxide dropwise, control the reaction temperature to 45°C, drop it in 3 to 4 hours, and keep it warm at 50°C for 2 Hour. After the reaction was completed, the reaction solution was transferred to a separatory funnel, and the water phase was separated from the oil phase, which contained 123.2 g (0.88 mol) of butanoneazine in the oil phase. Transfer the water phase into a four-necked flask equipped with a distillation device, a thermometer, a dropping funnel and agitation, heat in an oil bath, and steam out ammonia, methyl ethyl ketone and water (ammonia is absorbed with water, and the methyl eth...
Embodiment 2
[0060] Add 576g butanone, 90g formamide (2mol) and 408g ammonia water (mass fraction is 25%) in the four-neck flask that has reflux condenser, thermometer, dropping funnel and stirring, add mass fraction in the dropping funnel to be 50% 136g (2mol) of % hydrogen peroxide, heated to 40°C, added dropwise hydrogen peroxide, controlled reaction temperature to 40°C, dripped in 3-4 hours, then kept the reaction at 45°C for 2.5 hours after the dropwise addition. After the reaction was completed, the reaction solution was transferred to a separatory funnel, and the water phase was separated from the oil phase, which contained 245.4 g (1.75 mol) of butanoneazine in the oil phase. Transfer the water phase into a four-necked flask equipped with a distillation device, a thermometer, a dropping funnel and agitation, heat in an oil bath, and steam out ammonia, methyl ethyl ketone and water (ammonia is absorbed with water, and the methyl ethyl ketone is condensed and recovered), and the remai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com