Method for synthesizing N-substitued benzo-isothiazolone derivative
The technology of a kind of benzisothiazolinone and synthetic method is applied in the field of synthesis of N-substituted benzisothiazolinone derivatives, which can solve the problems of low yield of derivatives, low utilization rate of raw materials, easy water absorption and deliquescence, etc. Achieve high yield, stable product quality, and improve reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
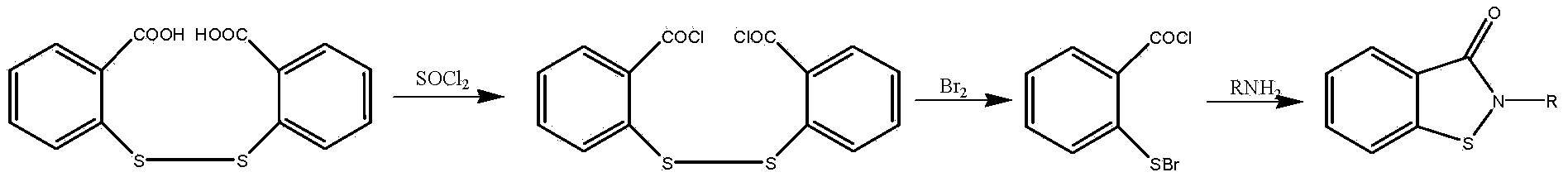
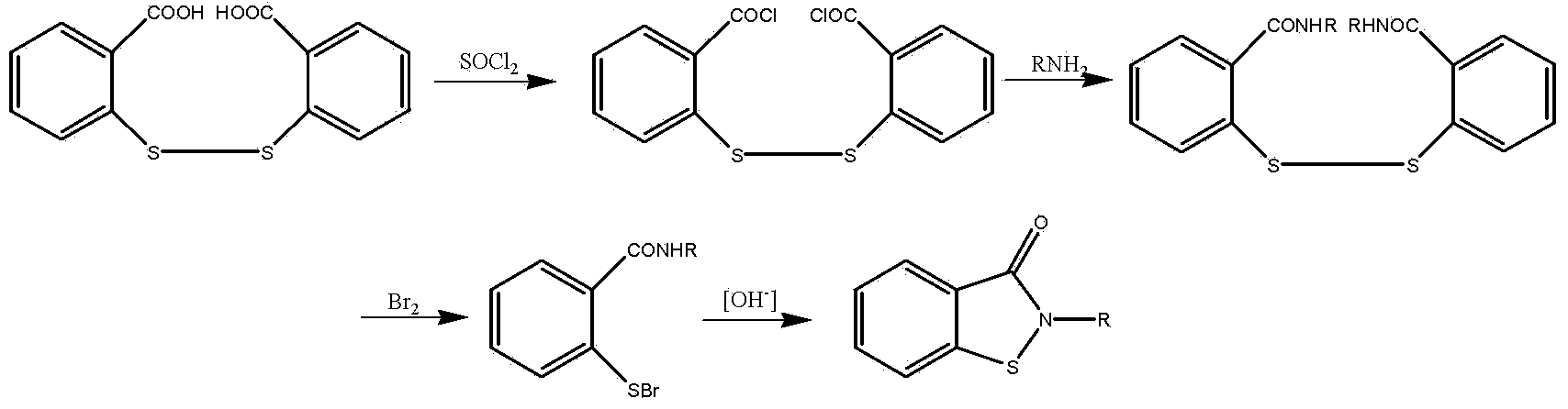
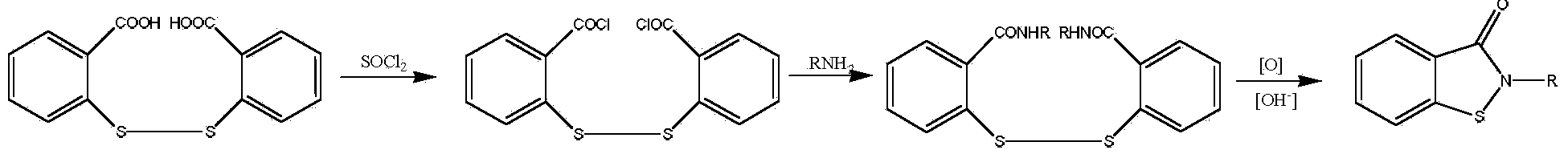
[0035] Step 1): Add 59.4g (0.2mol) triphosgene, 100g xylene, 6.1g (0.06mol) three Ethylamine, stir to dissolve, control the system temperature at -5~5°C, dissolve 91.8g (0.3mol) 2,2'-dithiodibenzoic acid in 200g xylene, and then drop it slowly and uniformly into the above-mentioned triphosgene In the mixed solution, the dropping time was controlled at 2 hours, then the temperature was raised to 100°C, the reaction was stirred for 3 hours, and 86.6 g of 2,2'-dithiodibenzoyl chloride was obtained by distillation under reduced pressure, with a yield of 84.2% and a purity of 98.4 %.
[0036] Step 2): Add 63.0g (0.6mol) aniline, 120ml xylene, 50.5g (0.5mol) triethylamine in a 500ml four-neck reaction flask equipped with a thermometer, agitator, reflux condenser, and constant pressure funnel Amine, stir to dissolve, control the temperature of the system at -5 ~ 5 ° C, slowly and uniformly drop into 150ml xylene solution dissolved with 86.6g (0.25mol) 2,2'-dithiodibenzoyl chloride, ...
Embodiment 2
[0039] Step 1): Add 59.4g (0.2mol) triphosgene, 150g chlorobenzene, 2.4g (0.03mol) pyridine to a 500ml four-necked reaction flask equipped with a thermometer, agitator, reflux condenser, and constant pressure funnel , stir to dissolve, control the system temperature at -5-5°C, dissolve 91.8g (0.3mol) 2,2'-dithiodibenzoic acid in 220g chlorobenzene, and then drop it slowly and uniformly into the above-mentioned triphosgene mixed solution , the dropping time was controlled at 2 hours, then the temperature was raised to 110°C, the reaction was stirred for 3 hours, and 89.2 g of 2,2'-dithiodibenzoyl chloride was obtained by distillation under reduced pressure, with a yield of 86.7% and a purity of 98.9%.
[0040]Step 2): Add 79.6g (0.624mol) 4-chloroaniline, 120ml xylene, 41.1g (0.52mol ) pyridine, stir to dissolve, control the temperature of the system at 0-5°C, slowly and uniformly drop into 150ml xylene solution dissolved with 89.2g (0.26mol) 2,2'-dithiodibenzoyl chloride, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com