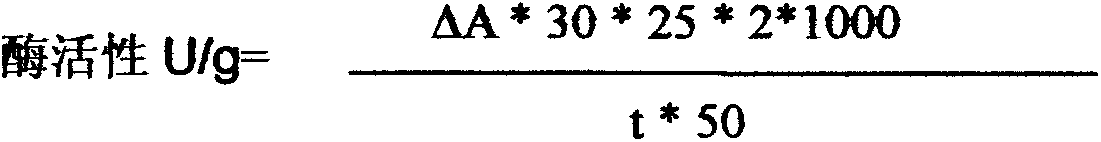Immobilized nuclease and preparing method thereof
A nuclease and solid-phase carrier technology, which is applied in the field of immobilized nuclease and its preparation, can solve problems such as unexpectedness, reduction of catalytic efficiency, and disturbance of microenvironment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The combination of embodiment one nuclease and agarose Sepharose-4B
[0059] Take an appropriate amount of Sepharose-4B gel, pump out the protective solution in a G3 sand core funnel, filter with a vacuum pump, weigh 50g of Sepharose-4B gel in wet weight, rinse with 500ml, 0.5mol / L NaCl solution, and remove the gel Clean the protective agent inside with distilled water.
[0060] In the fume hood, put on rubber gloves, carefully weigh 15g of BrCN, add 15ml of acetonitrile to dissolve it.
[0061] Transfer 50g of gel to a 500ml beaker, add 75ml of 0.2mol / L, pH10.0 Na 2 CO 3 -NaHCO 3 buffer, then place the beaker in an ice bath and stir slowly with a stirrer. Take a dropper and add BrCN solution dropwise to the beaker to make it react with Sepharose-4B gel, and take another dropper to add 6mol / L NaOH dropwise to the beaker to keep the pH in the reaction solution at about 10.0. Measured with a pH meter, continue to react for 5 minutes after adding BrCN. At this moment...
Embodiment 2
[0067] The combination of embodiment two nuclease and Sepharose CL-6B
[0068] Take 50mL of Sepharose CL-6B medium stored in 20% ethanol, wash repeatedly with excess distilled water to remove ethanol, then place it in a G3 sintered glass funnel and vacuum-dry it for 5min; weigh 10g of the medium after suction filtration, and use 20% 50% and 70% dimethyl sulfoxide (Dimethyl Sulfoxide, DMSO) aqueous solution cleaning, after each cleaning, vacuum in the cleaning solution; add 6.5ml2mol / L NaOH, 1,5ml epichlorohydrin to the treated medium (ECH), 15ml 56% 1,4-dioxane. The suspension of the medium was shaken and reacted at 170r / min for 2 hours on a constant temperature air bath shaker, and the reaction temperature was 45°C. After the reaction, it was washed with a large amount of deionized water until no epoxy group was detected in the cleaning solution. The epoxy group in the cleaning solution is measured by thiosulfate, using phenolphthalein as the indicator. If the cleaning solut...
Embodiment 3
[0072] The quantitative analysis of embodiment three epoxy group modification density
[0073] The epoxy-modified density of the agarose gel was determined by sodium thiosulfate titration. The activated medium after washing with deionized water was placed in a G3 funnel and vacuum-dried for 5 minutes, and then weighed 0.5 g and placed in a ground-mouth Erlenmeyer flask. Add about 3 mL of 1.3 mol / L sodium thiosulfate and 1-2 drops of phenolphthalein indicator, seal the Erlenmeyer flask and shake it at room temperature for 30 minutes. The reacted solution is titrated with 0.1 mol / L hydrochloric acid standard solution until the solution From red to colorless. According to the volume of the consumed hydrochloric acid standard solution, calculate the epoxy modification density: S=M*(V 0 -V 1 ) / W / ρ=91.2
[0074] Wherein: S is epoxy group modification density (mol / mL);
[0075] M is the concentration of HCl (mol / L);
[0076] V0, V1 is the volume (mL) of HCl before and after titra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com