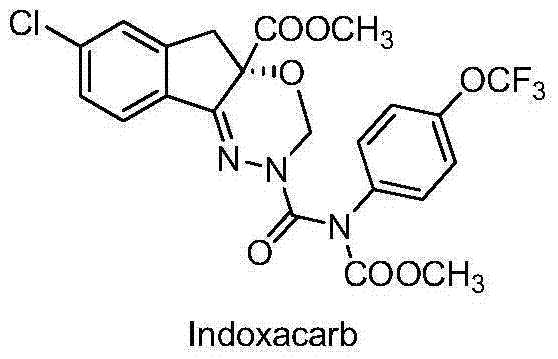
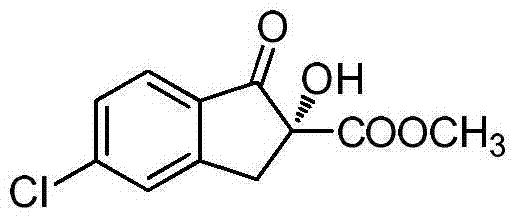
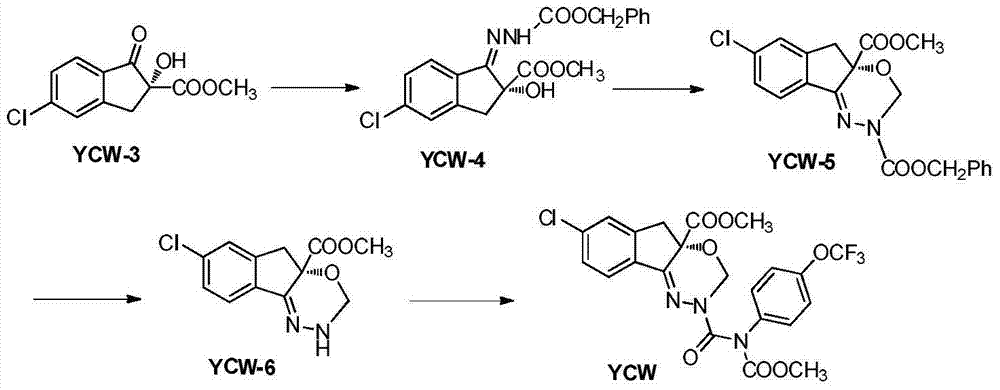
Method for synthesizing intermediate of agricultural insecticide indoxacarb
A technology for indoxacarb and intermediates, which is applied in the field of new synthetic techniques for intermediates of indoxacarb for agricultural insecticides, can solve problems such as low yields, and achieve the effects of increasing reaction temperature, reducing decomposition and improving overall yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, a kind of synthetic method of agricultural insecticide indoxacarb intermediate, carries out following steps successively:
[0041] 1) Add 10g of YCW-4 (0.026mol), 0.45g of p-toluenesulfonic acid (0.0026mol), and 50mL of toluene (with anhydrous treatment) into a 250mL four-neck flask with a fractionation device, and heat to reflux into the thorn-shaped fractionation column Droplets are formed, and the temperature in the four-necked bottle is T l is 108°C, the temperature T at the top of the thorn-shaped column 2 When the temperature is 106°C, start to intermittently add a mixed solution consisting of 10mL diethoxymethane (0.08mol) and 30mL toluene, the reflux temperature of the reaction solution will decrease during the dropwise addition, when the top temperature T 2When it is lower than 92°C, stop the dropwise addition, and start to separate part of the liquid to the temperature T at the top of the thorn-shaped column 2 Return to 92°C, stop the distillat...
Embodiment 2
[0043] Embodiment 2: a kind of synthetic method of agricultural insecticide indoxacarb intermediate, carry out following steps successively:
[0044] 1), add 10g YCW-4 (0.026mol), 0.83g ammonium diphenyltrifluoromethanesulfonate (0.0026mol), 50mL toluene (after anhydrous treatment) to a 250mL four-neck flask with a fractionation device, heat Droplets are generated in the backflow to the thorn-type reflux column, and the temperature T in the four-necked bottle is now l is 108°C, the temperature T at the top of the thorn-shaped column 2 When the temperature is 106°C, intermittently drop the mixed solution consisting of 10mL diethoxymethane and 30mL toluene, the reflux temperature of the reaction solution will decrease during the dropwise addition, when the top temperature T 2 When it is lower than 92°C, stop the dropwise addition, and start to separate part of the liquid to the temperature T at the top of the thorn-shaped column 2 Return to 92°C, stop the distillation operatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com