Recombinant plectasin as well as expression and purification method and application thereof
A technology of lectin and recombinant bacteria, applied in the biological field, can solve the problems of increasing the difficulty of curing diseases, patients and social and economic burden, and achieve the effects of large antibacterial spectrum, good bacteriostatic effect and wide antibacterial range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 recombine Plectasin prokaryotic recombine Construction of expression bacteria
[0038] (1) Design the nucleotide sequence of Plectasin as shown in SEQ ID NO.1.
[0039] (2) Construction of prokaryotic expression vector of recombinant plectasin
[0040] 1) Design and synthesis of plectasin nucleotide sequence as shown in SEQ ID NO.3, which was synthesized by Shanghai Jierui Bioengineering Co., Ltd.
[0042] The synthetic fragment (shown as SEQ ID NO.3) and the expression vector pE-SUMO were double digested with Bsa I and Xba I and then cloned to the same double digestion. The enzyme digestion system is as follows:
[0043]
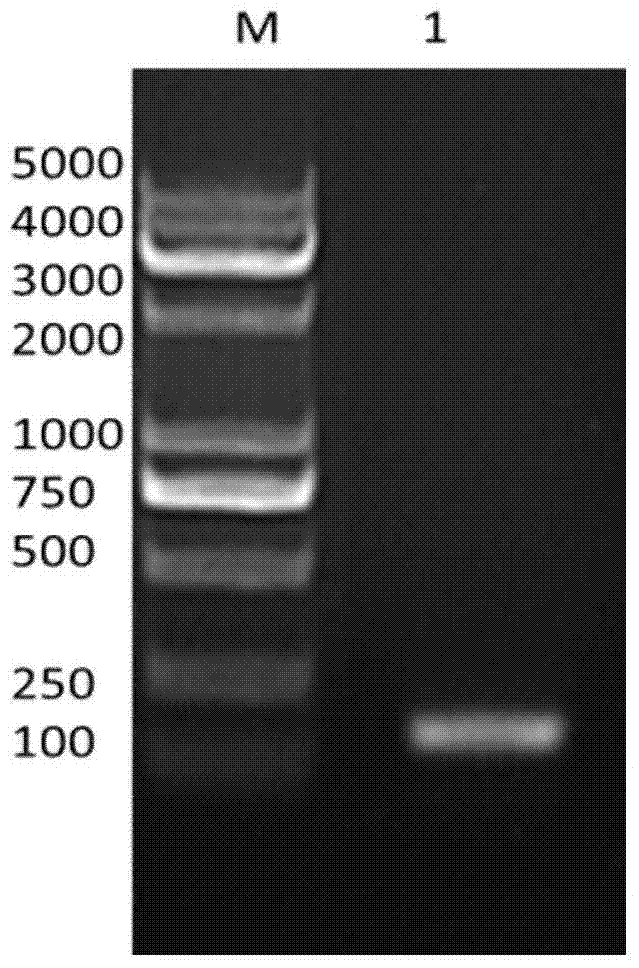
[0044] Digested at 37°C for 3 hours, electrophoresed on a 1% agarose gel and recovered using a gel recovery kit. After recovery, the target gene and the expression vector were ligated overnight at 16°C with T4 DNA ligase at a ratio of 3:1 to construct the recombinant expression vector pE-SUMO-Plecta...
Embodiment 2
[0052] Example 2 Plectasin fusion protein (recombinant plectasin) induced expression of
[0053] Pick the positive recombinant expression strain BL21(DE3) verified above and inoculate it in 500ml LB medium, cultivate it on a shaker at 37°C until OD600=0.5, and optimize it by orthogonal experiments with different concentrations of IPTG, different induction temperatures, and different induction times. The induction condition was 30°C, the rotational speed was 180rpm, the inducer used for induction expression was IPTG with a final concentration of 0.05mM, and the induction time was 6h to obtain a higher fusion protein, ie recombinant Plectasin.
Embodiment 3
[0054] Example 3 Plectasin fusion protein (recombinant plectasin) purification of
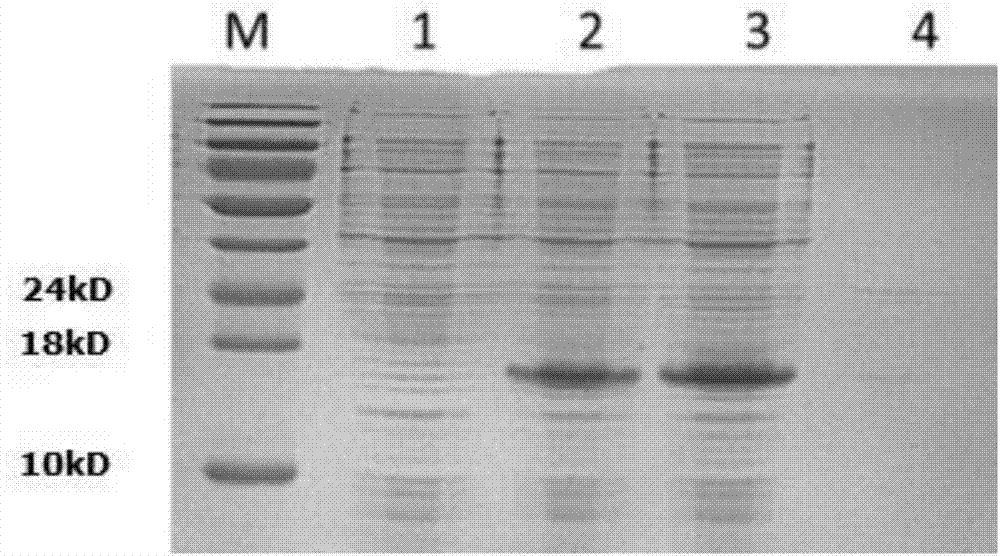
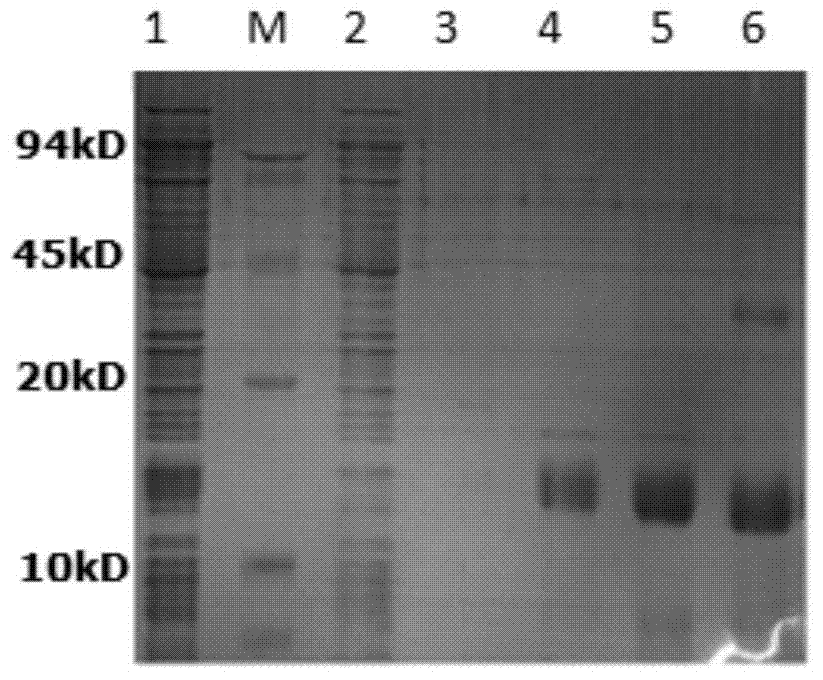
[0055] Collect the cells by centrifugation, resuspend the cells in 50ml crushing buffer SUMO1, and crush the cells by ultrasonic in an ice bath. Centrifuge at 14,000 rpm for 15 min at 4°C, collect the supernatant, and filter through a 0.22 μm filter. Use 15% SDS-PAGE to detect the protein distribution in the ultrasonic supernatant and precipitate, the protein detection results are as follows figure 2 As shown, M in the figure is the protein molecular weight standard; 1 is the total protein of the non-induced bacteria; 2 is the total protein of the induced bacteria; 3 is the supernatant of the induced bacteria sonicated; 4 is the sonicated precipitate of the induced bacteria. It can be seen that the fusion protein mainly exists in the supernatant with a molecular weight of about 16.7kDa. The total protein content in the ultrasonic supernatant was determined by Bradford method, and the gel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com