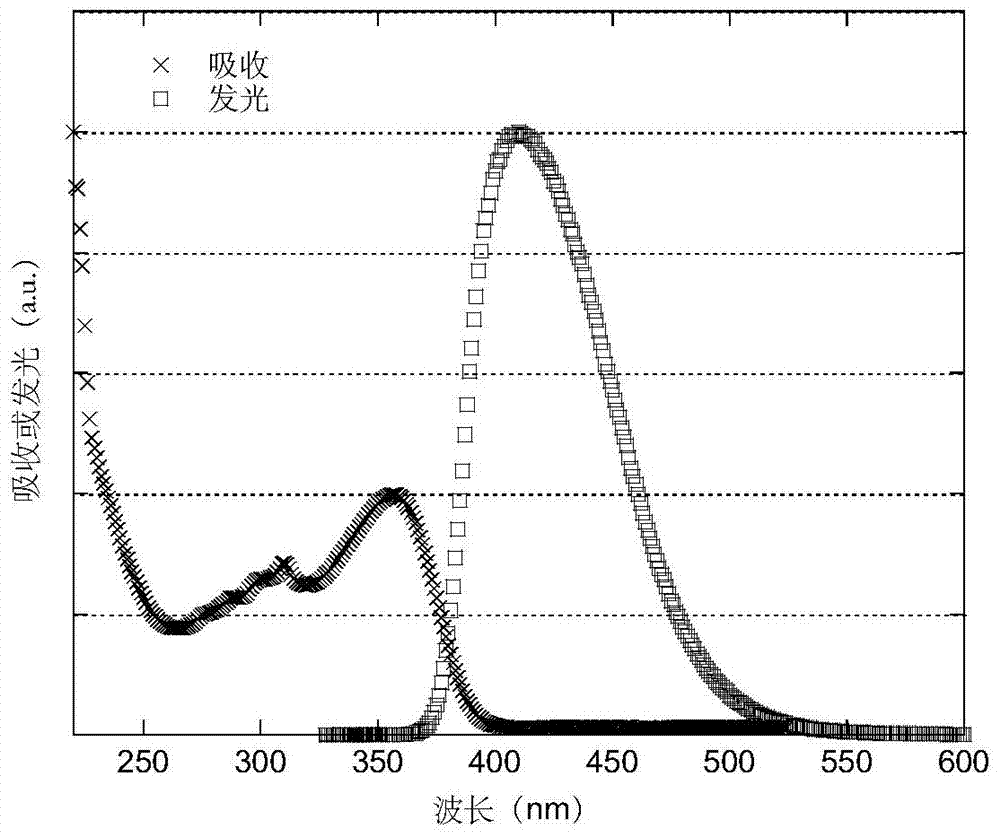
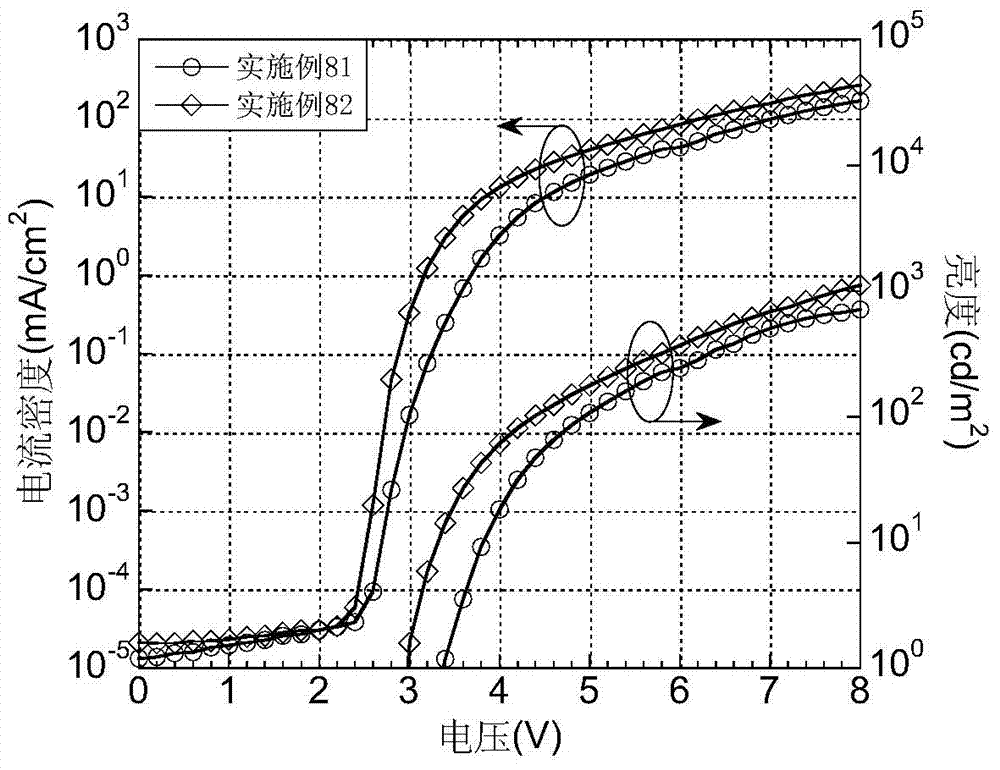
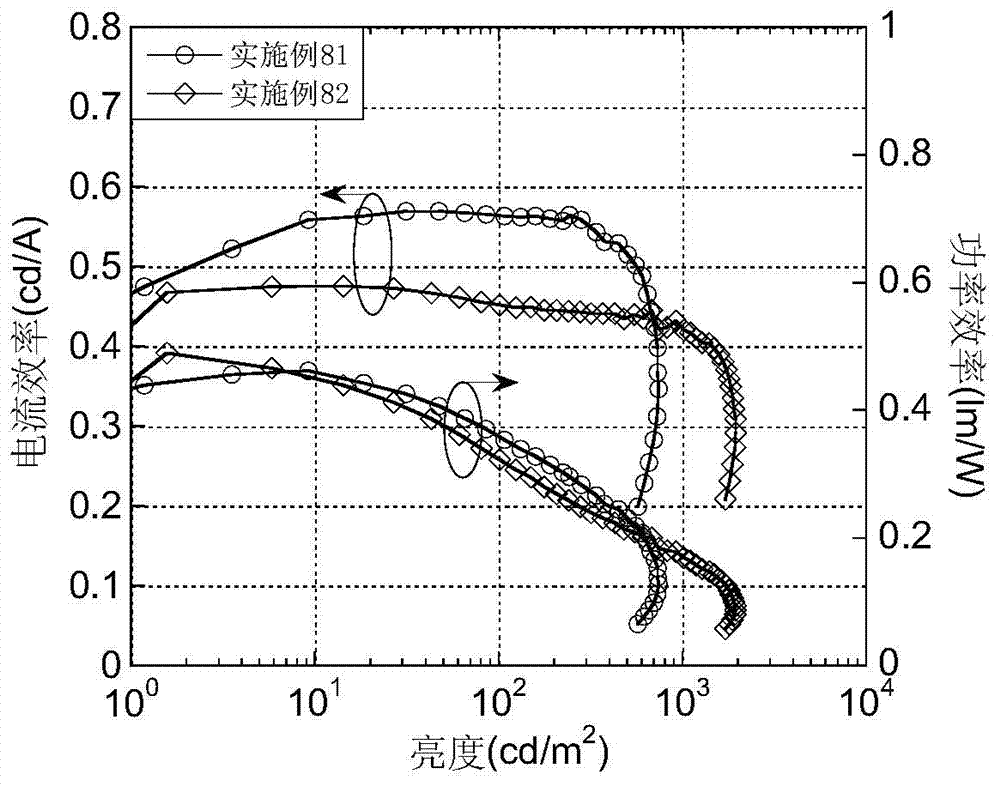
Luminescent materials based on thioxanthene-fluorene spiral structures and organic optoelectronic devices adopting the materials as luminescent layers
A technology for luminescent materials and optoelectronic devices, applied in luminescent materials, electrical solid devices, electrical components, etc., can solve the problems of organic light-emitting small molecules that are rarely reported, and achieve improved carrier transport characteristics, good reproducibility, and material structure single effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of intermediate 1 of this embodiment:
[0040] Add 4.66g (20mmol, 10equ) of 2-bromobiphenyl to the low-temperature reaction flask, dissolve it with 60mlTHF, and protect it with nitrogen gas. After sealing the device, add liquid nitrogen to cool to -78°C, and drop 8.0ml (2.5 M) Butyllithium, keep warm for 40min, then add 0.7g 3,7-dibromothioxanthone THF solution at one time, then react at room temperature overnight, the reaction is complete, remove THF with rotary evaporator, dichloromethane extraction, column separation The isolated product h1 was obtained, 20ml of acetic acid and an appropriate amount of hydrochloric acid were added to the isolated product, under nitrogen protection, stirred overnight at 80°C, and 10.83g of white solid intermediate 1 was obtained by column separation, with a yield of 82%. 1H NMR (400MHz, CDCl 3 , ppm): 7.86-7.82 (d, 2H), 7.50-7.54 (s, 2H), 7.25-7.40 (m, 4H), 7.02-7.05 (m, 4H), 6-95-7.00 (dd, 2H) . The reaction equati...
Embodiment 2
[0048] The preparation of intermediate 2 of this embodiment:
[0049]Dissolve 1.5 g of the intermediate 1 obtained in Example 1 with 30 ml of dichloromethane, add 12 ml of acetic acid and 3.0 ml of 30% hydrogen peroxide, and react overnight at 80° C. After the reaction is complete, extract with dichloromethane, dry over anhydrous magnesium sulfate, and the solid Sample loading, petroleum ether:dichloromethane=2:1 (volume ratio) was passed through the column, and 1.23 g of white solid intermediate 2 was isolated with a yield of 80%. 1H NMR (400MHz, CDCl 3 , ppm): 7.86-7.82 (d, 2H), 7.64-7.68 (dd, 2H), 7.50-7.54 (s, 2H), 7.38-7.43 (m, 4H), 7.25-7.40 (m, 4H). The reaction equation is as follows:
[0050]
Embodiment 3
[0052] The preparation of intermediate 3 of this embodiment:
[0053] Add 4.66g (20mmol, 10equ) of 2-bromobiphenyl to the low-temperature reaction flask, dissolve it with 60mlTHF, and protect it with nitrogen gas. After sealing the device, add liquid nitrogen to cool to -78°C, and drop 8.0ml (2.5 M) Butyllithium, keep warm for 40min, then add 0.582g of 3-bromothioxanthone THF solution at one time, then react overnight at room temperature, the reaction is complete, remove THF with rotary evaporator, extract with dichloromethane, column separation to obtain product h2 , adding 20ml of acetic acid and appropriate amount of hydrochloric acid to the product h2, under nitrogen protection, stirring at 80°C overnight, and column separation to obtain 0.65g of white solid intermediate product 3, with a yield of 76%. C 25 h 15 BrS M / S=426.01,, theoretical value: 428.01 (100.0%), 426.01 (99.0%), 429.01 (28.2%), 427.01 (27.7%), 428.00 (4.5%), 430.00 (4.4%), 430.01 (3.7 %), 431.00 (1.2%)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com