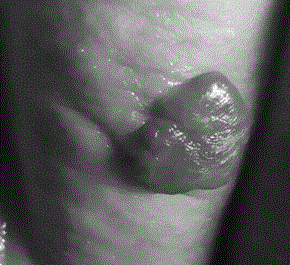
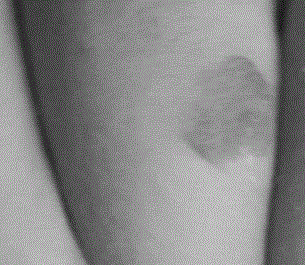
A long-acting timolol transdermal preparation and its application in hemangioma
A technology of timolol and transdermal preparation, which is applied to timolol long-acting transdermal preparation and its application in hemangioma, can solve the problems of large side effects, adverse reactions, scars left, etc. The effect of drug dosage, improving treatment effect, and promoting efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Preparation of timolol hydrogel long-acting transdermal preparation (1)
[0027] The small molecule drug timolol is prepared into a nano / micropowder hydrogel transdermal preparation, and the preparation process is as follows:
[0028] (1) Accurately weigh 10g of timolol and dissolve it in 100mL of water, then add 20g of porous silica nanoparticles, stir overnight to make timolol fully adsorbed in the silica particles, centrifuge to remove the supernatant, and then fully Washing, then freeze-drying to form timolol nano / micropowder medicine, the particle diameter of medicine is 20~100000 nanometers;
[0029] (2) Prepare a hydrogel solution with 12% by weight of polyvinyl alcohol (molecular weight: 40,000) and 1% by weight of polyethylene glycol (molecular weight: 400);
[0030] (3) Disperse the timolol nano / micropowder drug prepared in step (1) in the hydrogel solution prepared in step (2) according to the weight ratio of 1:1, and add benzene with a final weigh...
Embodiment 2
[0047] Example 2 Preparation of timolol nano hydrogel long-acting transdermal preparation (2)
[0048] The small molecule drug timolol is prepared into a nano-hydrogel long-acting transdermal preparation, and the preparation process is as follows:
[0049] (1) Accurately weigh 0.2g of timolol and dissolve it in 10mL of water, then add 1g of porous hydroxyapatite nanoparticles, stir overnight to make timolol fully adsorbed in the hydroxyapatite particles, and centrifuge to remove the supernatant , fully washed, and then freeze-dried to form timolol nano-medicine, the particle size of the nano-medicine is 20-500 nanometers;
[0050] (2) Prepare a hydrogel solution with 4% by weight of polyvinyl alcohol (molecular weight: 40,000), 4% by weight of polyvinyl alcohol (molecular weight: 136,000), and 2% by weight of polyethylene glycol (molecular weight: 400) ;
[0051] (3) Disperse the timolol nanomedicine prepared in step (1) in the hydrogel solution prepared in step (2) accordin...
Embodiment 3
[0057] Example 3 Preparation of timolol nano hydrogel long-acting transdermal preparation (3)
[0058] The small molecule drug timolol is prepared into a nano-hydrogel long-acting transdermal preparation, and the preparation process is as follows:
[0059] (1) Accurately weigh 10g of timolol and dissolve it in 100mL of water, then add 40g of porous silver nanoparticles, stir overnight to make timolol fully adsorbed in the silver nanoparticles, centrifuge to remove the supernatant, then fully wash, and then Freeze-drying to form timolol nano-medicine, the particle size of nano-medicine is 10-1000 nanometers;
[0060] (2) Prepare a hydrogel solution with 8% by weight of sodium carboxymethylcellulose, 5% by weight of polyvinyl alcohol (molecular weight: 100,000), and 1.5% by weight of polyethylene glycol (molecular weight: 400);
[0061] (3) Disperse the timolol nanomedicine prepared in step (1) in the hydrogel solution prepared in step (2) according to the weight ratio of 1:10,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com