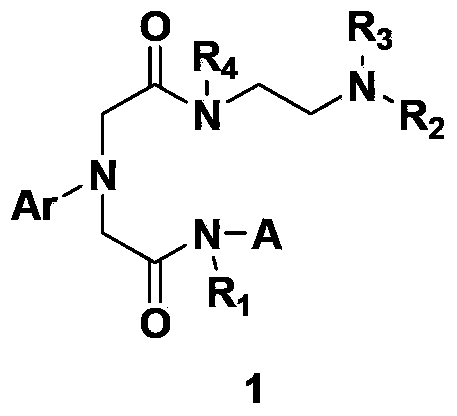
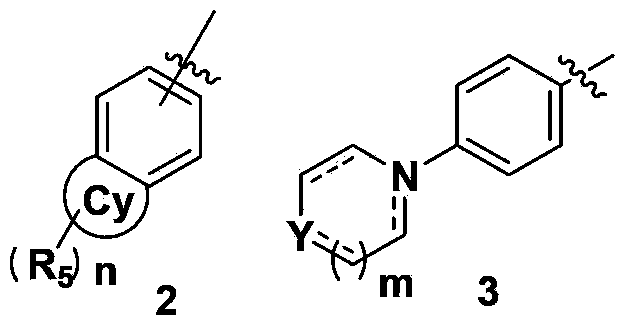
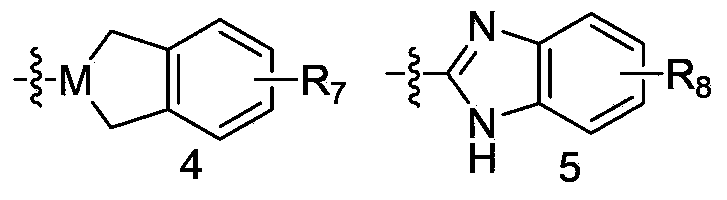Glycinamide derivative, and intermediate, preparation method, pharmaceutical composition and application thereof
A compound and pharmaceutical technology, applied in the fields of drug combination, bulk chemical production, cardiovascular system diseases, etc., can solve problems such as large side effects, poor therapeutic effect, and poor safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0593] Example 1 Compound 20 Preparation of (Method 1)
[0594] 1-methyl-5-nitro-1H-indazole and 2-methyl-5-nitro-2H-indazole preparation of
[0595] Put 17.6g of NaH with a mass percentage of 60% (the mass percentage refers to the percentage of the mass of sodium hydride in the total mass of sodium hydride reagent) and 100mL DMF into the three-necked flask, stir and cool to 0°C, and slowly add Mixture of 60g 5-nitroindazole and 200mL DMF, control the reaction temperature below 10°C, after the dropwise addition, react at room temperature for 30 minutes, then cool to 0°C, slowly add dropwise a mixture of 62.68g methyl iodide and 60mLDMF solution, after the dropwise addition was completed, the reaction was carried out at room temperature for 12 hours. Add 1000mL water to the reaction solution, extract with 400mL×3 dichloromethane, combine the organic phases, wash twice with water, once with saturated NaCl, and wash with anhydrous MgSO 4 Dry and concentrate to dryness to ...
Embodiment 2
[0596] Example 2 Compound 19 preparation of
[0597] Preparation of 1-methyl-1H-indazol-5-amine
[0598] Drop into 60g1-methyl-5-nitro-1H-indazole in there-necked flask, 1200mL ethanol, 3g mass percent is 10% Pd / C (the described mass percent refers to the quality of palladium to the total mass of palladium-carbon reagent %), stirred at room temperature (25°C) and continuously fed H 2 After bubbling (about 10.08g), react for 12 hours, filter, and concentrate to obtain 49.9g of brown-yellow solid, yield 100%. ESI-MS(m / z):148.09[M+H] + .
Embodiment 3
[0599] Example 3 Compound 20 preparation of
[0600] Preparation of 1-(4-nitrophenyl)-1H-pyrrole (Method 2)
[0601] Put 60g of 4-fluoronitrobenzene, 88.15g of potassium carbonate, 400ml of DMSO and 57.05g of pyrrole into the three-necked flask, stir and heat up to 90°C, and react for 5 hours. After the reaction is completed, pour the reaction solution into 1500ml of water, and a large amount of The white solid was filtered, and the obtained solid was dried at 45° C. to obtain 64 g of white solid, with a yield of 80.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com