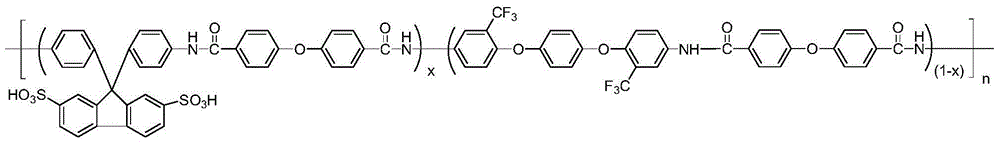
Sulfonated aromatic polyamide containing fluorenyl and trifluoromethyl structures and preparation method thereof
A technology for sulfonating polyaramide and trifluoromethyl is applied in the field of aromatic sulfonated polymers and their preparation, which can solve the problems of complex synthesis and preparation process, high fuel permeability, low service temperature and the like, and achieves simple synthesis process. , the effect of high proton conductivity and good chemical oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
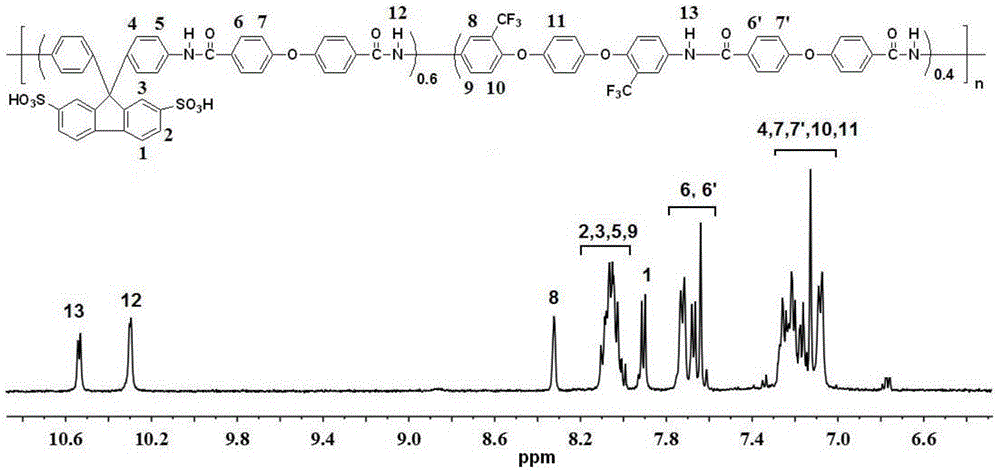
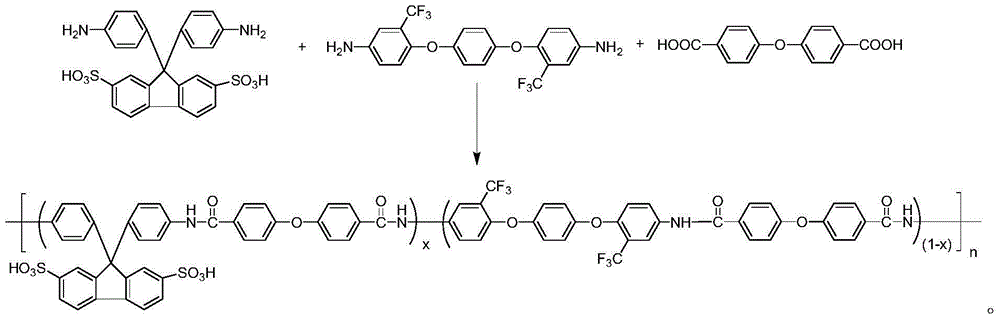
[0020] In a 100ml dry three-necked flask equipped with nitrogen protection and a condenser tube, add 0.6103g (1.2mmol) of 9,9-bis(4-aminophenyl)fluorene-2,7-disulfonic acid (to sulfonate Structural unit content x=0.6 as an example), 0.3427g (0.8mmol) of 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene, 0.5165g (2mmol) of 4,4' -diphenyl ether diacid, 1.2412g (4mmol) of triphenyl phosphite, 0.3164g (4mmol) of pyridine, 0.4048g (4mmol) of triethylamine, 0.2220g (2mmol) of calcium chloride and 6.3ml of N,N -Dimethylacetamide, after the co-condensation reaction at 130°C for 4 hours, pour it into ethanol to obtain a fibrous solid polymer, further soak it in 1mol / L hydrochloric acid solution for 12 hours, filter and dry to obtain the product, The yield was 97% (based on the conversion rate of 9,9-bis(4-aminophenyl)fluorene-2,7-disulfonic acid); 1 H NMR (DMSO, 400MHz) as attached figure 1 shown.
Embodiment 2
[0022] In a 100ml dry three-necked flask equipped with nitrogen protection and a condenser tube, add 0.6103g (1.2mmol) of 9,9-bis(4-aminophenyl)fluorene-2,7-disulfonic acid (to sulfonate Structural unit content x=0.6 as an example), 0.3427g (0.8mmol) of 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene, 0.5165g (2mmol) of 4,4' -diphenyl ether diacid, 2.4824g (8mmol) of triphenyl phosphite, 0.6328g (8mmol) of pyridine, 0.8096g (8mmol) of triethylamine, 0.6660g (6mmol) of calcium chloride and 14.1ml of N,N -Dimethylacetamide, after the co-condensation reaction at 100°C for 8 hours, pour it into ethanol to obtain a fibrous solid polymer, further put it into 1mol / L sulfuric acid solution and soak it for 24 hours, filter and dry to obtain the product, The yield was 96% (based on the conversion of 9,9-bis(4-aminophenyl)fluorene-2,7-disulfonic acid).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com