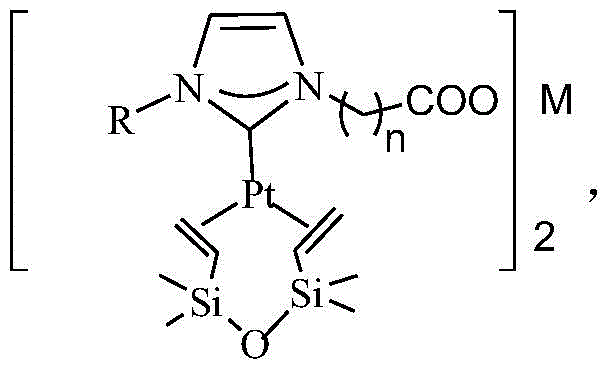
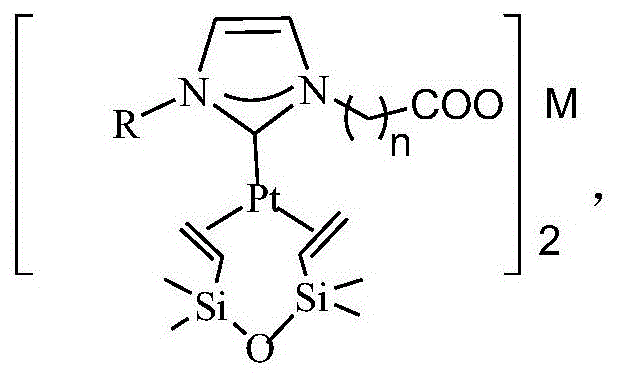
A kind of n-heterocyclic carbene platinum complex carboxylate metal salt integrated catalyst and preparation method thereof
A carboxylate metal salt and catalyst technology, which is applied to the N-heterocyclic carbene platinum complex carboxylate metal salt integrated catalyst and the field of preparation thereof, can solve the problems of easy generation of platinum black, low selectivity and the like, and achieve easy separation, The effect of high recycling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation of catalyst and the steps of olefin hydrosilylation reaction are:
[0033] Add alkylimidazole and 1-chlorocarboxylic acid in a molar ratio of 1:1 to a round bottom flask equipped with magnetic stirring, heating and condensing devices, add trichloroethane as a reaction solvent, and at 60-90°C, Stir the reaction for 8h, cool down, pour off the supernatant, wash twice with ethyl acetate, remove the residual solvent under reduced pressure, and use it directly for the next reaction. The above prepared carboxylic acid functional imidazolium salt is dissolved in dehydrated alcohol, according to the ratio of alkylimidazole / metal chloride molar ratio of 2:1, adding ferrous, copper, zinc, calcium or magnesium chloride, at 60 At -90°C, stir the reaction for 3 hours, remove the solvent under reduced pressure, and suspend the obtained solid in anhydrous tetrahydrofuran. The imidazole molar ratio is 1:1) and stirred for 1 h, and the solvent was removed to obtain the ...
Embodiment 1
[0042] In a 100 ml three-necked flask, 1.64 g of 1-methylimidazole, 1.88 g of chloroacetic acid, and 20 ml of trichloroethane were added, the temperature was raised to 80° C., the reaction was stirred for 8 h, and cooled to room temperature. Trichloroethane was removed by decantation, washed with ethyl acetate (25 mL×2), and the residual solvent was removed under reduced pressure. The solid was dissolved with 20 ml of absolute ethanol, and 1.99 g of FeCl was added 2 4H 2 O, stirred at 80°C for 3h, removed ethanol by rotary evaporation, and dried in a vacuum oven at 70°C for 24h. The obtained solid was suspended in 25 ml of anhydrous tetrahydrofuran, 2.24 g of potassium tert-butyl alkoxide was added, stirred for 30 minutes, 39 g of Karstedt catalyst with a platinum content of 10% was added, stirred for 1 h, and the solvent was removed to obtain N-heterocyclic carbene platinum complex carboxylate Acid metal salt integrated catalyst.
[0043] Take 0.15 mmol of the catalyst, 1 ...
Embodiment 2
[0046] Take 0.15 mmol of the catalyst of Example 1, 1 mol of 1-hexene and 1.1 mol of triethoxyhydrogensilane in a closed container containing a magnetic stirrer, and react in a silicone oil bath at 80°C for 4 hours, and the conversion rate of 1-hexene is 99.6%. , β-addition product selectivity was 99.9%.
[0047] Separate the upper layer product, add another 1mol 1-hexene and 1.1mol triethoxyhydrogensilane, react under the same conditions for 4h, the conversion rate of 1-hexene is 99.0%, and the selectivity of β-addition product is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com