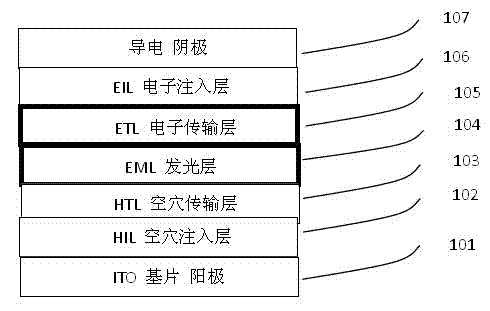
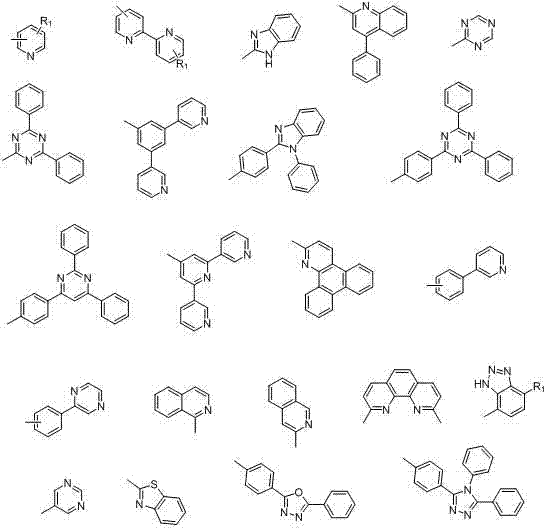
Heat-resistant organic electronegative semiconductor
A technology of organic semiconductors and compounds, applied in the field of heat-resistant organic electronegative semiconductors, which can solve the problems of short lifespan of heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
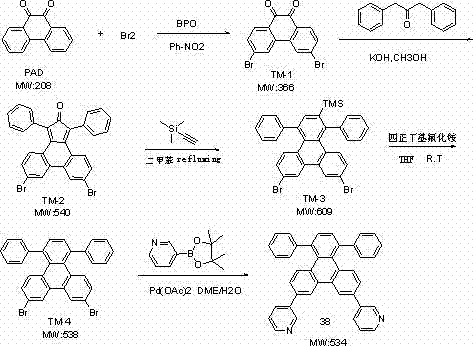
[0086] Example 1: Synthesis of electron transport material 38
[0087]
[0088] Synthesis of compound TM-1: Add 20.50g of phenanthrenequinone, 48g of bromine, 1.0g of benzoyl peroxide and 300ml of nitrobenzene to reflux for 2-3 hours, cool to room temperature, filter, wash the solid with 400ml of ethanol and dry to obtain 29.21g of TM- 1, the yield is 82%;
[0089] Synthesis of compound TM-2: Heat 29.21g TM-1, 18.44g dibenzyl ketone, 5.00g potassium hydroxide and 350ml methanol, heat up to 50°C, react for 5-6 hours, cool down to room temperature, filter, wash the solid with water first, then wash with methanol Washing, drying obtains 32.5g TM-2, and yield is 76%;
[0090] Synthesis of compound TM-3:With 32.5g of TM-2, 9.2g of trimethylsilylacetylene and 250ml of xylene, the reaction was refluxed overnight, then lowered to room temperature, filtered, and the solid was purified by column to obtain 16.86g of TM-3 with a yield of 46% ;
[0091] Synthesis of compound...
Embodiment 2
[0093] Example 2: Synthesis of electron transport material 45
[0094]
[0095] Synthesis of Compound 45: 1.00g TM-4, 2.22g Pienalate , 0.10g palladium acetate, 0.20g S-Phos, 1.32g of potassium carbonate, 15ml of ethylene glycol dimethyl ether and 10ml of water, nitrogen replacement, then warming up to reflux, then cooling to room temperature, splitting, and passing the crude product through the column to obtain 1. 0g of product compound 45, yield: 60%, product characterization: Tg=200 degrees, PL=417nm, MS=370 degrees.
Embodiment 3
[0096] Example 3: Synthesis of electron transport material 51
[0097]
[0098] Synthesis of compound 51: 1.00g TM-4, 2.00 Pienalate , 0.10g palladium acetate, 0.20g S-Phos, 1.32g of potassium carbonate, 15ml of ethylene glycol dimethyl ether and 10ml of water, nitrogen replacement, then warming up to reflux, then cooling to room temperature, splitting, and passing the crude product through the column to obtain 0.9g of product 51, yield: 64%, product characterization: Tg=193 degrees, PL=418nm, MS=400 degrees.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com