Preparation method of amorphous dasatinib
A dasatinib and amorphous technology, which is applied in the field of producing amorphous dasatinib, can solve problems such as numerous operation steps, loss of dasatinib, complex refining methods, etc., and achieves low process pollution, complete dissolution, and easy operation The effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The invention provides a preparation method of amorphous dasatinib, which firstly dissolves the raw materials of various crystal forms of dasatinib with anhydrous solvent and co-solvent, and then uses spray drying and cyclone separation methods to rapidly precipitate dasatinib The solid powder of tinib is then dried under reduced pressure to remove the solvent to obtain the dasatinib amorphous product whose residual solvent complies with the Chinese Pharmacopoeia. The method of the present invention is described in detail below:
[0035] (1) Dissolution of various crystal forms of dasatinib
[0036] Add the raw materials of various crystal forms of dasatinib into a certain amount of solvent, and add a certain amount of co-solvent, stir and heat to 60-100°C to dissolve until the raw materials of dasatinib are completely dissolved. The solvent should be an anhydrous organic solvent that is soluble in dasatinib, such as ethanol, isopropanol, acetone, etc. The mass ratio ...
Embodiment 1
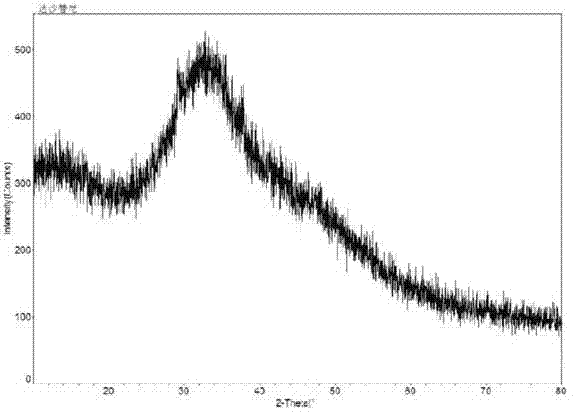
[0048] Add 10 g of dasatinib raw material to 100 g of ethanol, and add 0.1 g of DMF as a cosolvent. Heat at 80°C to dissolve, enter Buqi B-290 spray dryer while it is hot, control hot air heating temperature at 100-150°C, hot air outlet temperature at 80-100°C, control vacuum degree to less than -0.095MPa, flow control and cyclone separation and collection It is suitable for solid powder. After spray-drying, the collected solid powder enters a decompression oven, heats and dries at 80°C for 6 hours under high vacuum, further dries, and removes the solvent to obtain Dasatinib amorphous solid with a yield of 99.2%, purity and addition The Dasatinib raw material is relatively unchanged, and the X-ray diffraction pattern of the powder is as follows: figure 1 shown. from figure 1 Looking at it, no sharp diffraction angle peaks were found, proving that the product is amorphous dasatinib. After the Karl Fischer moisture test, the moisture content is less than 2%. As determined b...
Embodiment 2
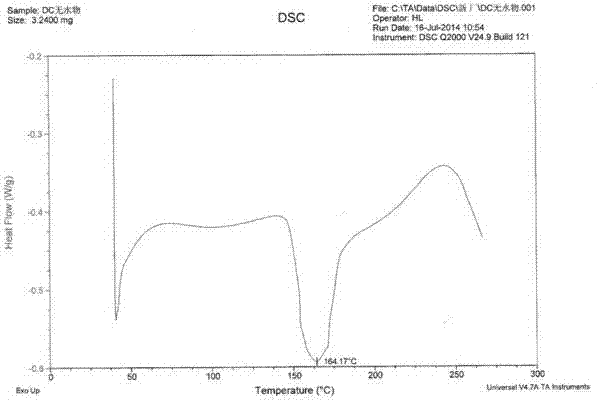
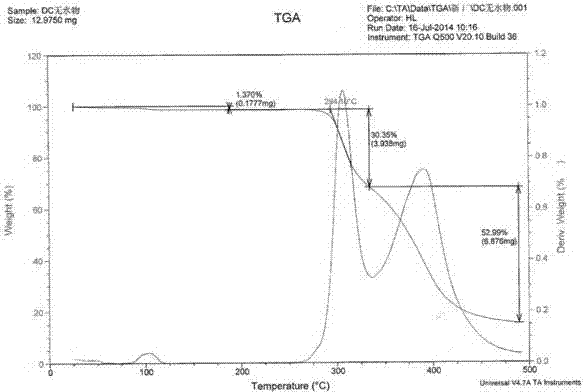
[0053] 10 g of dasatinib raw material was added to 150 g of isopropanol, and 0.1 g of DMSO was added as a cosolvent. Heat at 80°C to dissolve, enter Buqi B-290 spray dryer while it is hot, control hot air heating temperature at 100-150°C, hot air outlet temperature at 80-100°C, control vacuum degree to less than -0.095MPa, flow control and cyclone separation and collection It is suitable for solid powder. After spray-drying, the collected solid powder enters a decompression oven, heats and dries at 80°C for 8 hours under high vacuum, further dries, and removes the solvent to obtain Dasatinib amorphous solid with a yield of 99.6%, purity and addition The raw material dasatinib was relatively unchanged. From the X-ray diffraction pattern, combined with the DSC and TGA patterns, it can be known that the prepared dasatinib is amorphous dasatinib. After the Karl Fischer moisture test, the moisture content is less than 2%. As determined by headspace gas chromatography, the residu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com