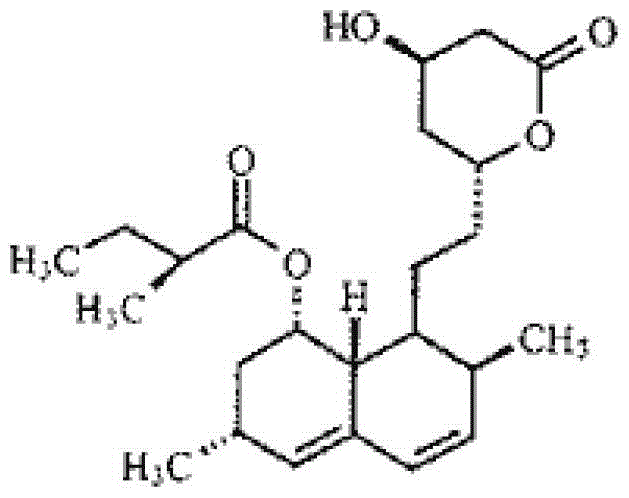
Method for recovering lovastatin from lovastatin crystallization mother liquor
A technology of lovastatin and crystallization mother liquor, which is applied in the field of lovastatin production technology, can solve the problems of lengthy and complicated operation process, high safety and environmental protection pressure, and high solvent consumption, and achieve simple operation steps, improved recovery rate, and accelerated recovery process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Take 1000ml of lovastatin crystallization mother liquor, wherein the lovastatin unit is 54666U / ml, add 400ml of 0.4mol / L sodium bicarbonate solution, stir and wash for 30 minutes, after stirring and washing is completed, let stand, separate the water phase, and obtain the organic phase 975ml; add 400ml of 0.4mol / L sodium bicarbonate solution to the organic phase, stir and wash for 30 minutes, let it stand, separate the water phase, and obtain 965ml of the organic phase; add 965ml of 1% (w / v, that is, dissolve 1g of oxalic acid in 100ml of water to configure 1% oxalic acid aqueous solution, the same below) was stirred and washed for 1 hour, left to stand, and the aqueous phase was separated to obtain 963 ml of organic phase. Add 100ml of 2% oxalic acid ethanol solution (2g of oxalic acid in 100ml of absolute ethanol) to the washed organic phase for cyclization treatment at 80°C for 2 hours, then properly concentrate at 73°C and vacuum degree ≤ -0.09Mpa , after standing f...
Embodiment 2
[0113] Take 2000ml of lovastatin crystallization mother liquor, wherein the unit of lovastatin is 54696U / ml, add 750ml of 0.4mol / L sodium bicarbonate solution and stir and wash for 30 minutes, after stirring and washing is completed, let stand, separate the water phase to obtain the organic phase 1980ml; add 1980ml of 1% oxalic acid aqueous solution, stir and wash for 1 hour, let stand, separate the water phase, and obtain 1960ml of the organic phase. Add 58.8ml of glacial acetic acid to the washed organic phase, cyclize at 80°C for 2 hours, concentrate properly at 75°C, vacuum degree ≤ -0.09Mpa, stand for crystallization for 8 hours, and filter with suction to obtain lovastatin The crude product is 87.8g, the content is 73.2%, and the yield is 80.26%.
Embodiment 3
[0115] Take 1000ml of lovastatin crystallization mother liquor, wherein the lovastatin unit is 50696U / ml, add 300ml of 0.4mol / L sodium bicarbonate solution, stir and wash for 30 minutes, after stirring and washing is completed, let stand, separate the water phase, and obtain the organic phase 980ml; add 980ml of 1% oxalic acid aqueous solution, stir and wash for 1 hour, let stand, separate the water phase, and obtain 965ml of the organic phase. Add 9.7ml of acetic acid to the washed organic phase, cyclize at 80°C for 2 hours, concentrate properly at 75°C under vacuum ≤ -0.09Mpa, stand for crystallization for 8 hours, and filter with suction to obtain Loval The crude statin was 39.62g, the content was 73.8%, and the yield was 78.15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com