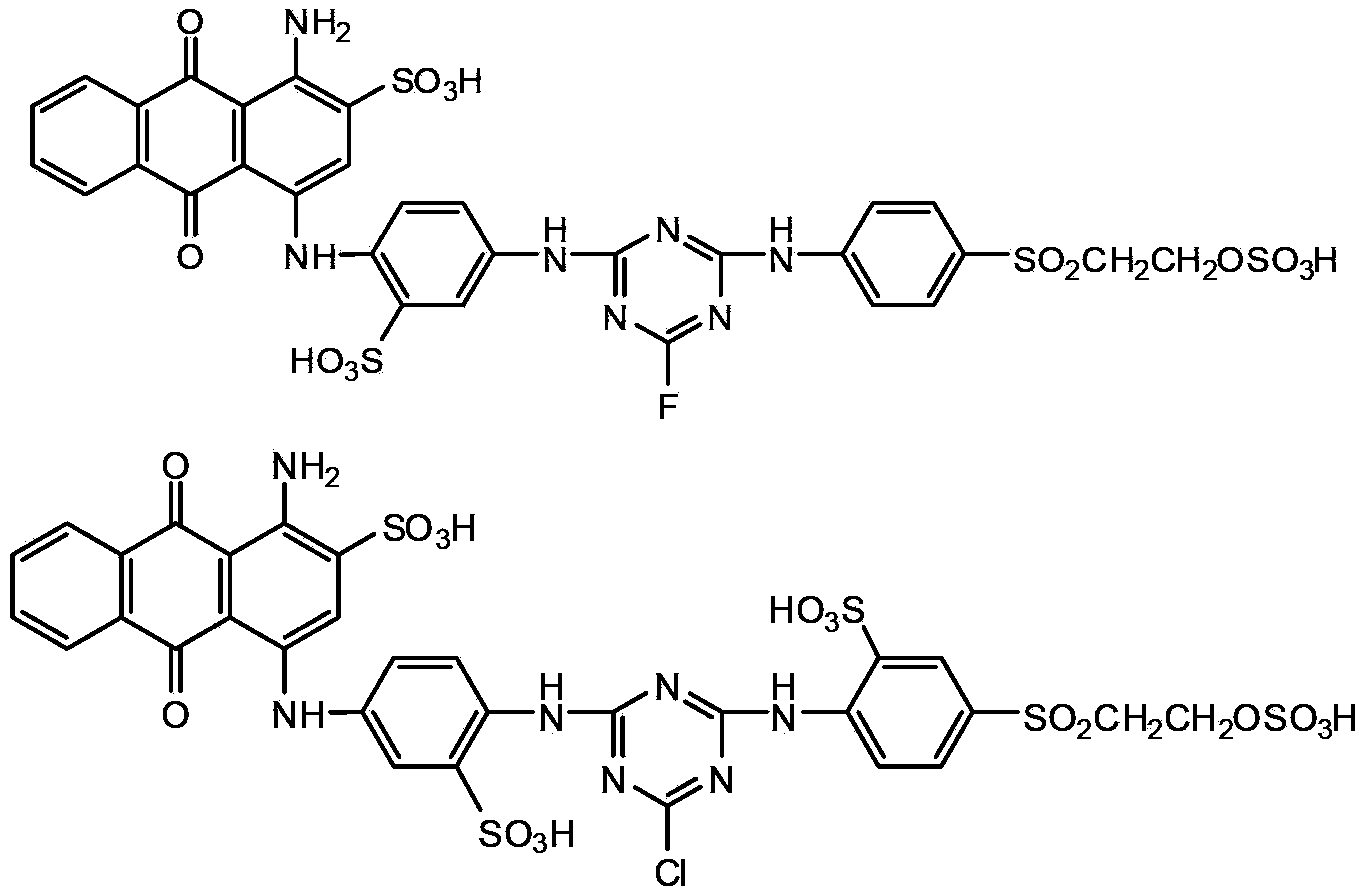
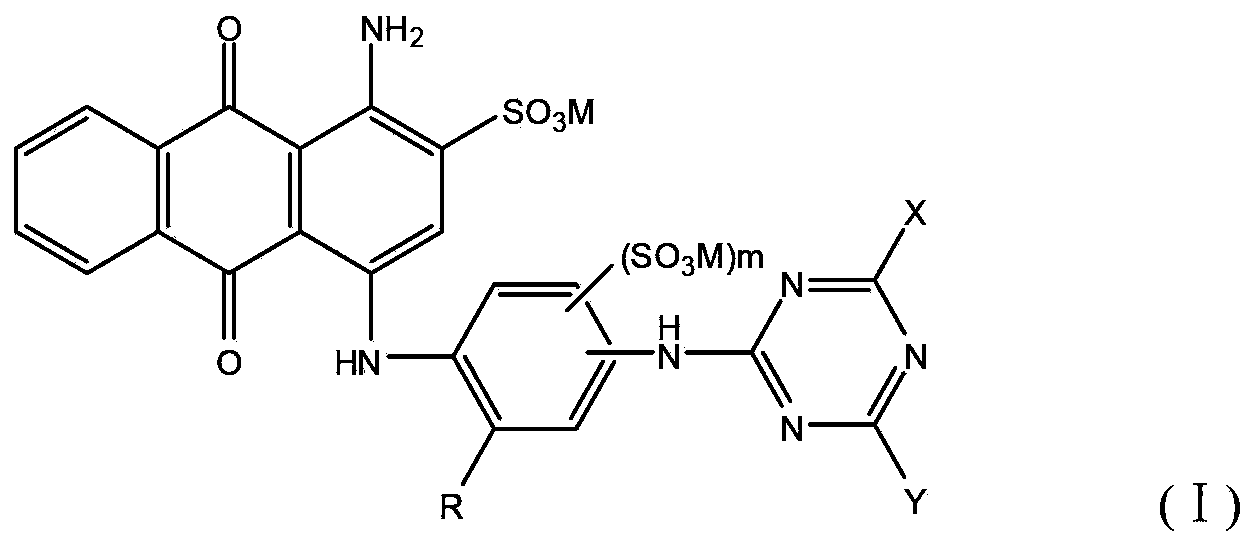
Reactive brilliant blue dye and preparation method thereof
A technology of reactive brilliant blue and dye, applied in the field of reactive brilliant blue dye and its preparation, can solve the problems of dull shade, poor fastness, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Prepare the following dyes:
[0073]
[0074] a, One condensation reaction of cyanuric chloride and p-beta-hydroxyethylsulfone aniline sulfate
[0075] Add 1.85 parts of cyanuric chloride and 10 parts of water into a three-necked reaction bottle, and stir and beat for 20 minutes in an ice-water bath. In addition, 2.81 parts of p-β-hydroxyethylsulfone aniline sulfate was weighed, mixed with 10 parts of water, dissolved with alkali, and then added to the cyanuric chloride solution. The reaction temperature is 0-15°C, the pH is between 3-6, and the reaction is followed by HPLC until the p-β-hydroxyethylsulfone aniline sulfate disappears, and a condensate solution is obtained, which is set aside.
[0076] b, the synthesis of compound E
[0077]
[0078] Add 3.4 parts of sodium bicarbonate, 2 parts of sodium carbonate and 40 parts of water into the three-necked reaction flask, and stir to dissolve. After completely dissolving, add 8.08 parts of bromonic acid, 4.36 p...
Embodiment 2-14
[0085] With the method described in similar embodiment 1, select following corresponding raw material for use, can obtain the dyestuff shown below:
[0086]
[0087]
[0088]
[0089]
Embodiment 15
[0091] Prepare the following dyes:
[0092]
[0093] Adjust the pH of the dicondensation solution obtained in Example 1 to 10 with sodium hydroxide, and keep the temperature at 40-50°C for reaction, and follow the reaction with HPLC until the dicondensate disappears. Adjust the pH to 7.0 with 30% hydrochloric acid solution, cool down to 10°C in ice water, collect the filter cake by filtration, and dry to obtain 8.5 parts of the reactive dye Brilliant Blue product of the above-mentioned dye (15).
[0094] Dyeing the above-mentioned dye (15) on polyamide fibers yields a bright blue color with a color absorption rate of 98.8% and a color fixation rate of 97.3%.
[0095] The synthesized dye (15) compound was subjected to high-resolution mass spectrometry, the calculated dye molecular weight M=768.02, and the measured dye molecular weight M=767.95. The calculated value is [M-1] / 1=767.02, and the measured value is 766.95; the calculated value is [M-2] / 2=383.01, and the measured ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color fastness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com