Method for synthetizing carbamate
A carbamate and synthetic method technology, applied in the field of synthetic carbamate, can solve the problems of threatening human safety, high toxicity and explosion, environmental pollution, etc., and achieve the reduction of environmental hazards, non-toxic raw materials, and environmental friendliness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
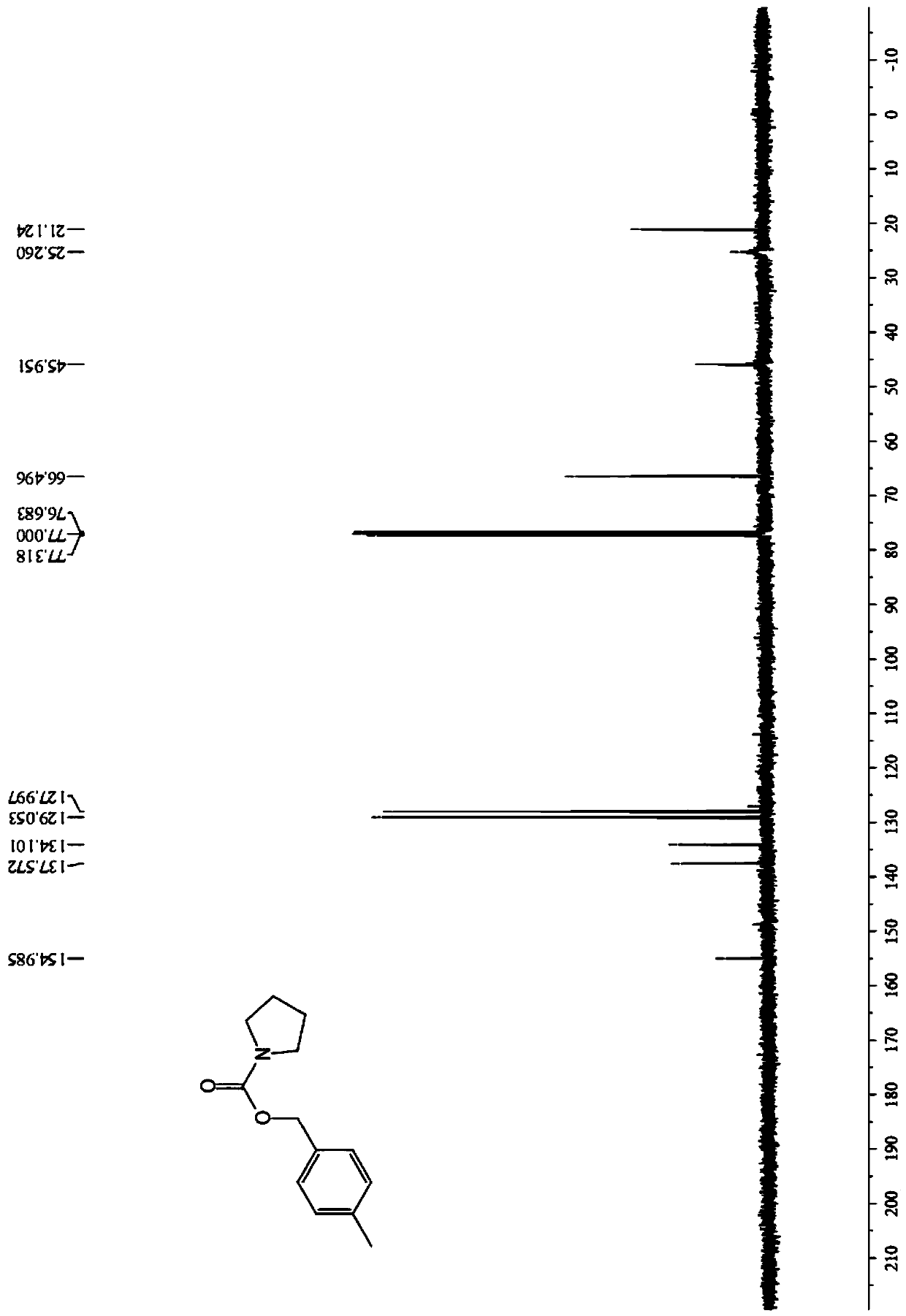
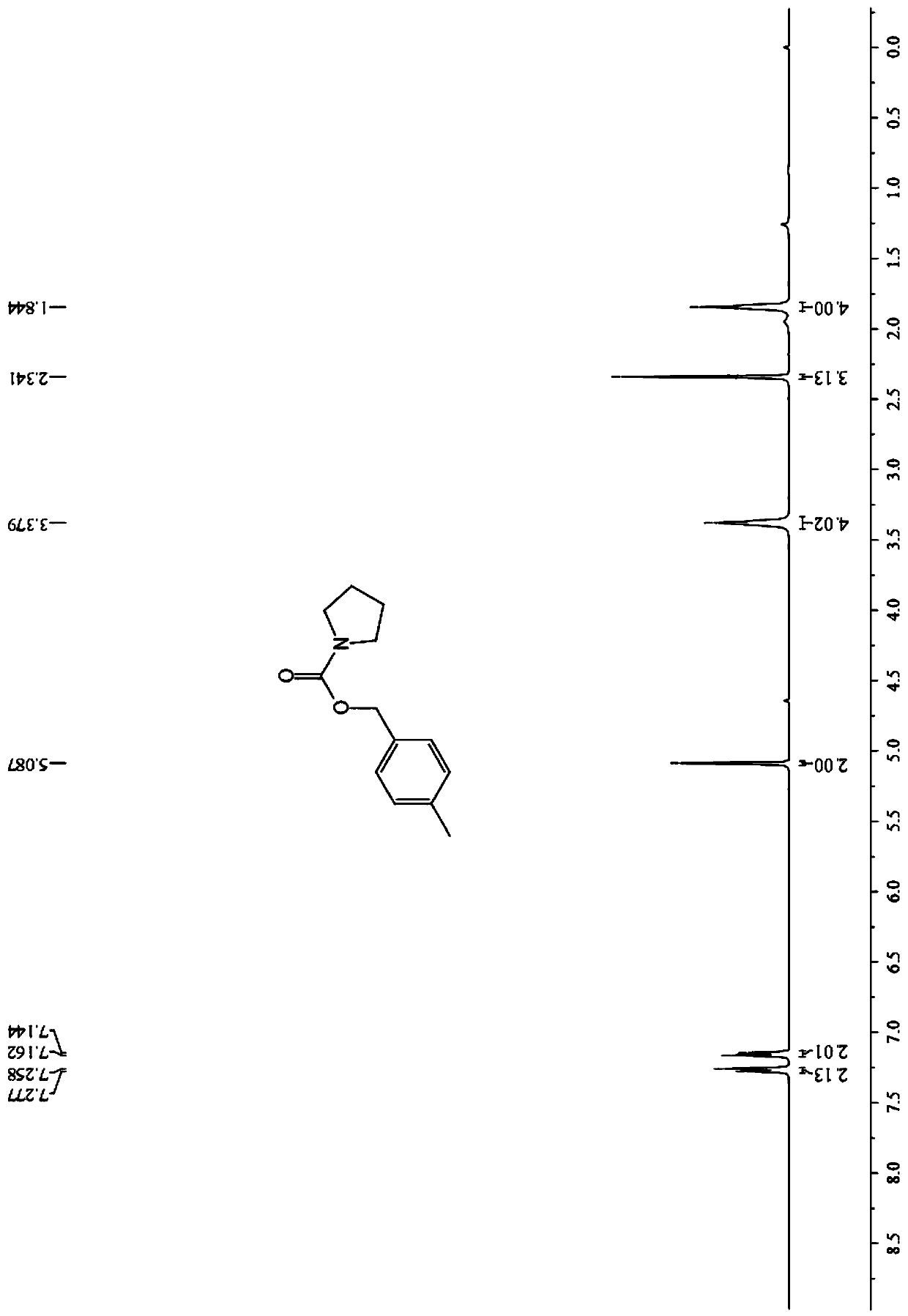
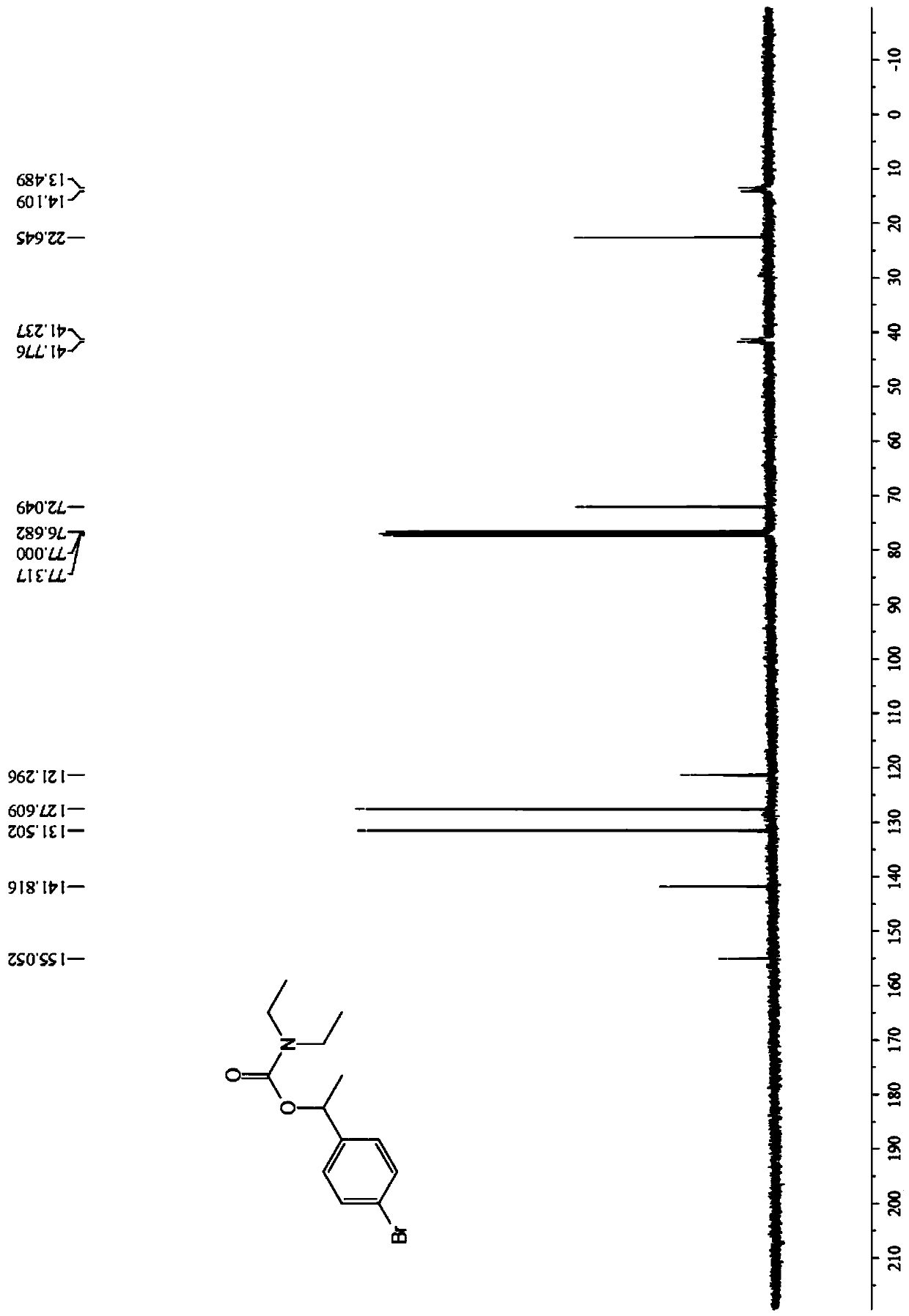
Image
Examples
Embodiment 1
[0042] Add 0.5 mmol of p-bromoacetophenone p-toluenesulfonylhydrazone, 0.5 mmol of tetrahydropyrrole, 0.5 mmol of potassium carbonate, 3 ml of acetonitrile, and fill with 0.5 MPa of CO in the autoclave. 2 , after stirring and reacting at 50°C for 6 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. Rate 8%.
Embodiment 2
[0044] Add 0.5 mmol of p-bromoacetophenone p-toluenesulfonylhydrazone, 10 mmol of tetrahydropyrrole, 2.5 mmol of potassium carbonate, 6 ml of acetonitrile, and fill with 10 MPa of CO in the autoclave. 2 , after stirring and reacting at 150°C for 72 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2. The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. rate 58%.
Embodiment 3
[0046] Add 0.5 mmol of p-bromoacetophenone p-toluenesulfonylhydrazone, 5 mmol of tetrahydropyrrole, 1 mmol of potassium carbonate, 3 ml of acetonitrile, and fill with 6 MPa of CO in the autoclave. 2 , after stirring and reacting at 120°C for 24 hours, stop heating and stirring, cool to room temperature, and slowly vent unreacted CO 2 . The reaction solution was filtered, and the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product. rate 49%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com