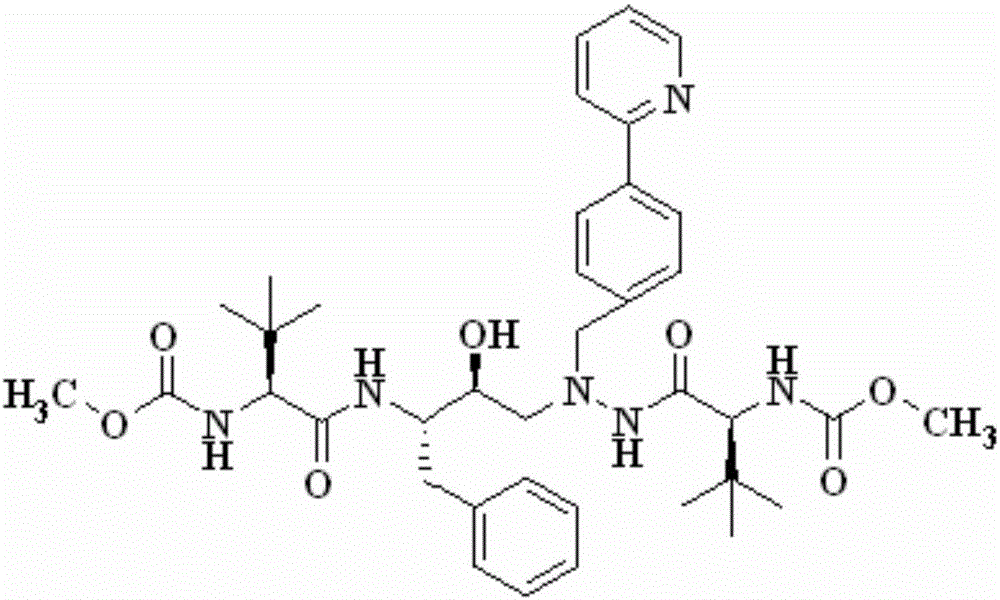
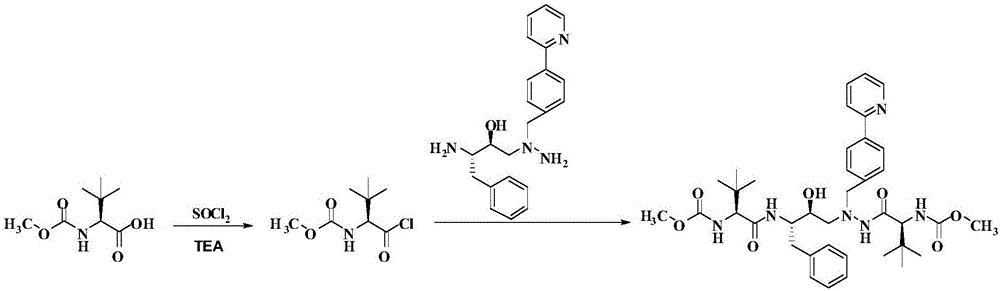
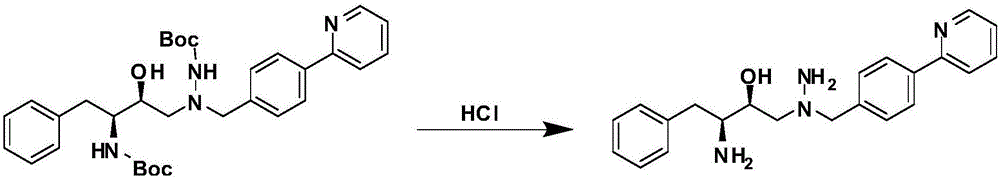
A kind of method for preparing atazanavir monomer
A monomer and phenyl technology, applied in the field of preparation of atazanavir monomer, can solve the problems of eye and skin irritation, complex separation and purification of products, unsuitable for industrial production, etc., achieve easy separation and purification, and high product yield , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-diamino-6-phenyl-2-azepine Preparation of alkane
[0019] In a clean reaction flask, add 27.45g (48.8mmol) of 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-bis[ (tert-butoxycarbonyl)amino]-6-phenyl-2-azidine and 45mL of dichloromethane, add 22.7g of hydrochloric acid dropwise; after dropping, raise the temperature to 40-45°C and keep it for about 3h; TLC analysis and tracking , the reaction was completed, lowered to room temperature, added 24.4g triethylamine, stirred at this temperature for 2h, layered, the organic layer was dried with anhydrous magnesium sulfate for 2h, filtered, the filter cake was rinsed with a small amount of solvent, and concentrated to dryness under reduced pressure , to obtain 16.92 g of solid product, yield 96.1%.
Embodiment 2
[0020] Embodiment 2: the preparation of atazanavir monomer
[0021] Put 18.9 g (100.0 mmol) of N-methoxycarbonyl-L-tert-leucine in a 500 mL eggplant-shaped bottle, add 200 mL of dichloromethane and stir to dissolve it, then add 70.5 g and 14.2 g of triethylamine ( 112.0 mmol) of thionyl chloride, the temperature was raised to 42°C and the solvent dichloromethane was refluxed, and the temperature was kept for 3 hours. After the reaction was completed, the temperature was lowered to room temperature, and the solid triethylamine hydrochloride was removed by filtration, and the filtrate was set aside.
[0022] Dissolve 32.58 g (90.0 mmol) of the solid product in Example 1 in 50 mL of dichloromethane, slowly drop the filtrate in the above step into the system at room temperature, stir overnight, and the reaction is complete. The reaction solution was successively washed with 10% citric acid, 10% potassium carbonate, 10% sodium chloride and purified water (300mL×2), concentrated und...
Embodiment 3
[0023] Example 3: 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-diamino-6-phenyl-2-azepine Preparation of alkane
[0024]In a clean reaction flask, add 41.175g (73.2mmol) of 1-[4-(pyridin-2-yl)-phenyl]-4(S)-hydroxyl-5(S)-2,5-bis[ (tert-butoxycarbonyl)amino]-6-phenyl-2-azepine and 80mL of ethyl acetate, add 36.3g of hydrochloric acid dropwise; after dropping, raise the temperature to 50°C and keep it for about 2.5h; TLC analysis tracking, After completion of the reaction, drop to room temperature, add 35.1g N-methylmorpholine, stir at this temperature for 2h, separate layers, dry the organic layer with anhydrous sodium sulfate for 2h, filter, rinse the filter cake with a small amount of solvent, and concentrate under reduced pressure to Dry to obtain 25.17g of solid product, yield 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com