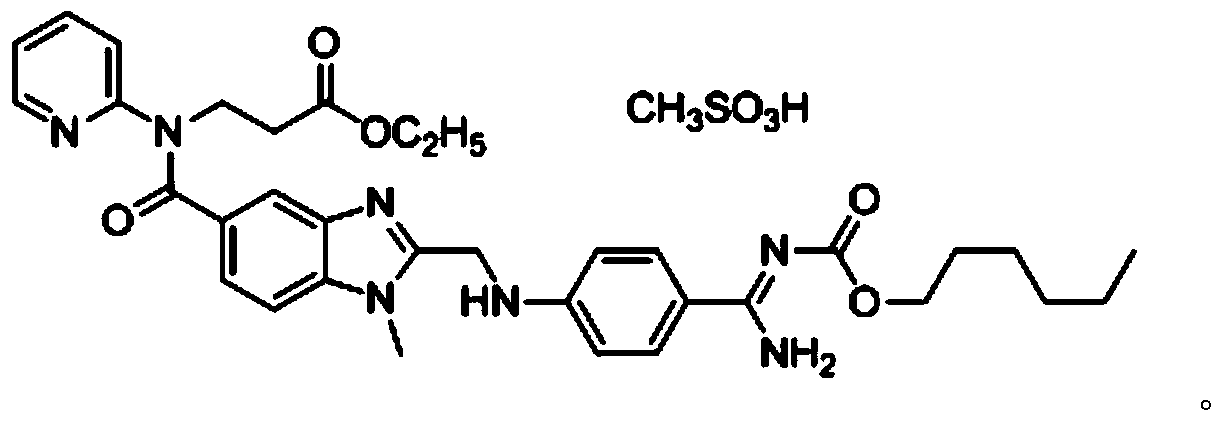
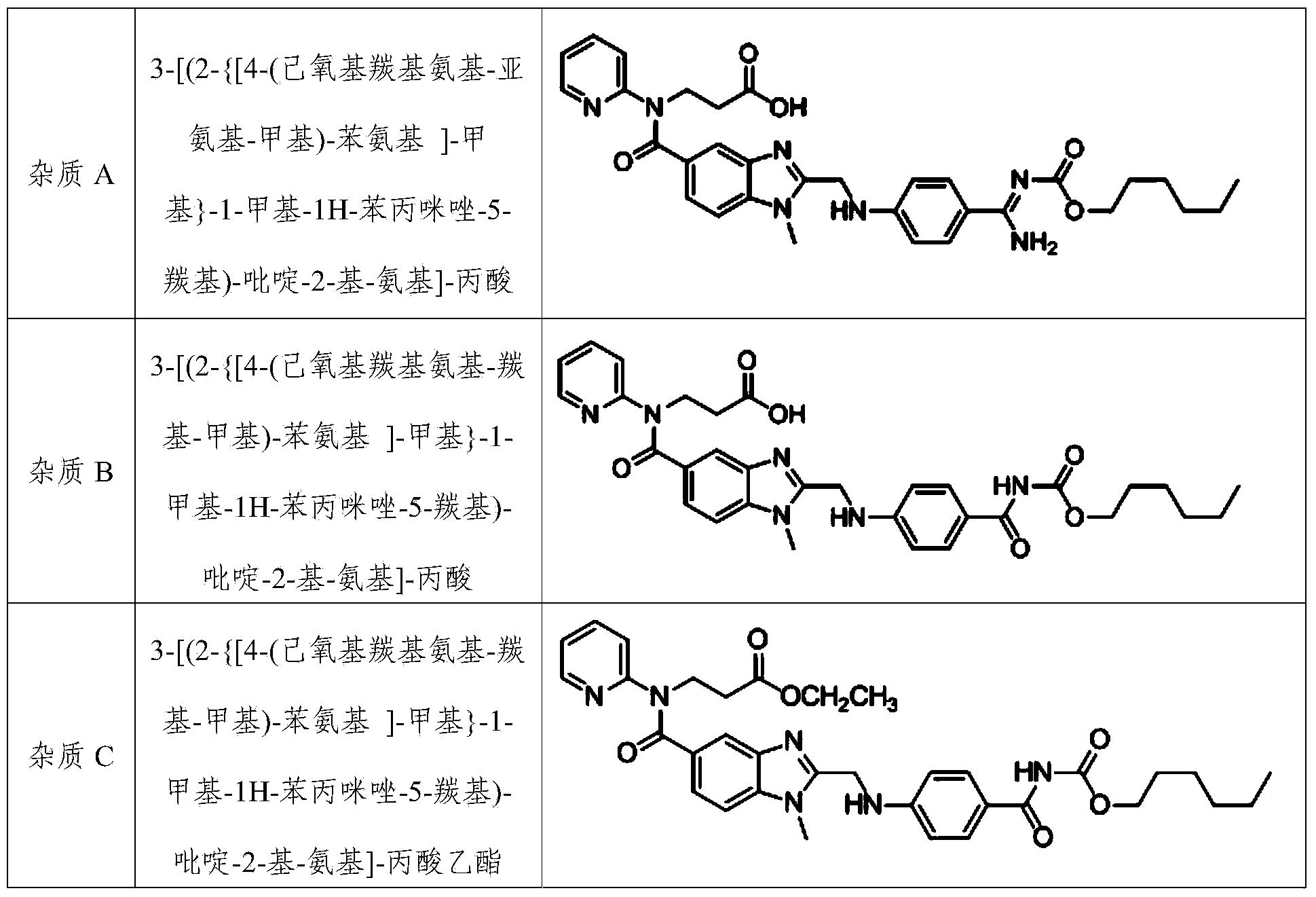
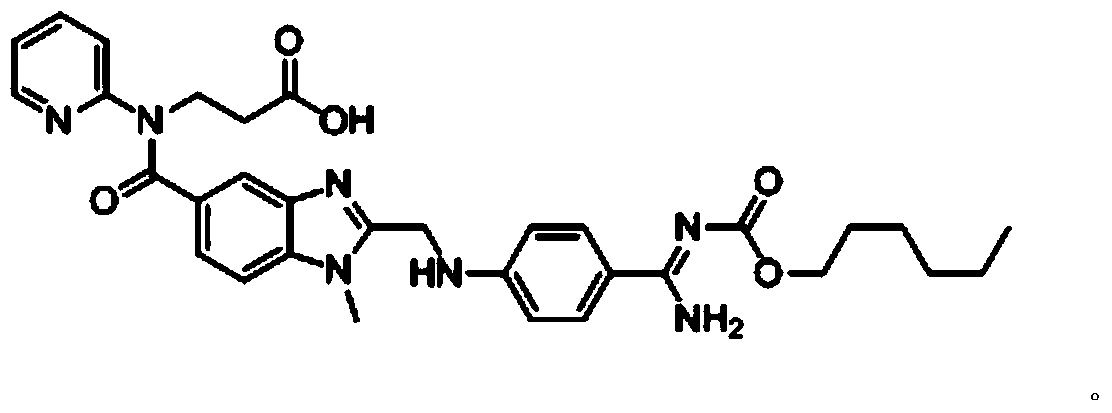
Method for preparing dabigatran etexilate hydrolysis impurities
A technology of dabigatran etexilate and impurities, applied in the field of preparation of dabigatran etexilate hydrolyzed impurities, to achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Prepare impurity A according to the following steps:
[0067] (1) Take 2g of dabigatran etexilate and dissolve it in 30mL of acetonitrile and 30mL of 0.1mol / L sodium hydroxide aqueous solution, and carry out the hydrolysis reaction at 20°C. After the reaction is complete, a mixed solution is obtained;
[0068] (2) Adjust the mixed solution obtained in step (1) to pH 4.0 with acetic acid, precipitate an oily substance, discard the solvent, and wash the oily substance twice with distilled water;
[0069] (3) Add 20 ml of acetone to the oil obtained in step (2), fully stir to dissolve, stand still to precipitate crystals, remove the solvent by suction, and dry in vacuo to obtain 1.04 g of yellow solid powder with a weight yield of 52%.
[0070] Product detection: After ESI(+)-MS mass spectrometry detection, the product obtained in this example has [M+H] + Is 600.7, [M+Na] + The peak is 622.7, which is related to dabigatran etexilate impurity A (C 32 H 37 N 7 O 5 ) Is consistent wit...
Embodiment 2
[0072] Prepare impurity A according to the following steps:
[0073] (1) Take 2g of dabigatran etexilate and dissolve it in 45mL of acetonitrile and 30mL of 2mol / L sodium carbonate aqueous solution, and carry out the hydrolysis reaction at 50℃. After the reaction is complete, a mixed solution is obtained;
[0074] (2) Adjust the mixed solution obtained in step (1) to pH 3.5 with acetic acid, precipitate an oily substance, discard the solvent, and wash the oily substance twice with distilled water;
[0075] (3) Add 20ml of isopropanol to the oil obtained in step (2), fully stir to dissolve, stand still to precipitate crystals, remove the solvent by suction, and dry in vacuo to obtain 0.74 g of light yellow solid powder, with a weight yield of 37%; After testing, the obtained powder is impurity A, with a purity of 98.5%.
Embodiment 3
[0077] Prepare impurity A according to the following steps:
[0078] (1) Take 2g of dabigatran etexilate and dissolve it in 20mL of ethanol and 40mL of sodium carbonate aqueous solution with a concentration of 0.02mol / L, and carry out the hydrolysis reaction at 10°C. After the reaction is complete, a mixed solution is obtained;
[0079] (2) Adjust the mixed solution obtained in step (1) to pH 4.5 with acetic acid, precipitate an oily substance, discard the solvent, and wash the oily substance twice with distilled water;
[0080] (3) Add 40ml of acetone to the oil obtained in step (2), fully stir to dissolve, leave to stand to precipitate crystals, remove the solvent by suction, and dry in vacuo to obtain 0.83g of light yellow solid powder, with a weight yield of 41.5%; , The resulting powder is impurity A, with a purity of 97.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com