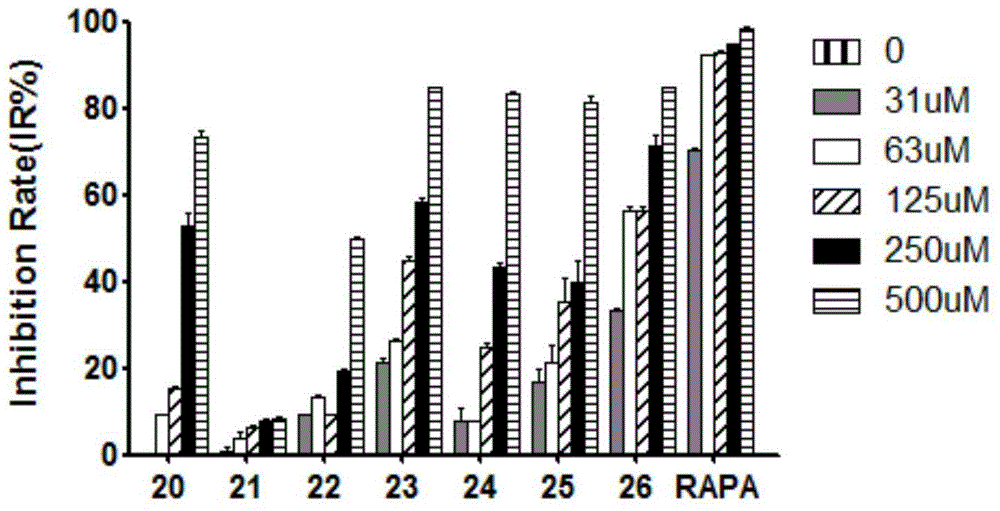
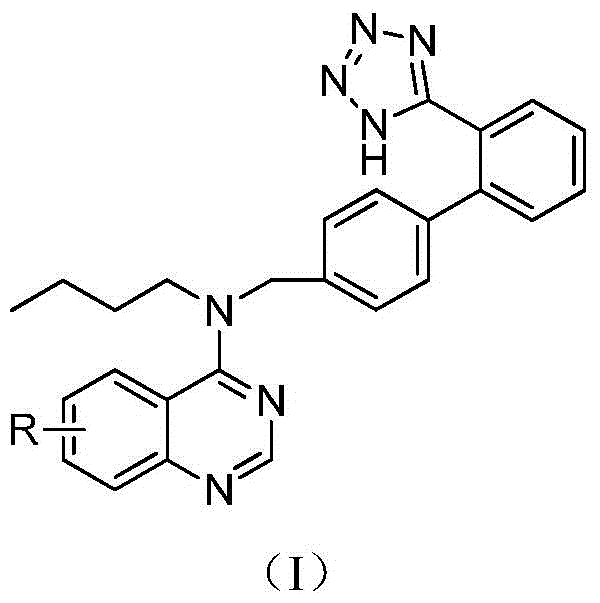
A class of quinazoline compounds and their application as immunosuppressants
A kind of quinazoline, immunosuppressive technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 4-{N-butyl-N-[(1H-tetrazol-5-yl]biphenyl)methyl]amine}-6-methoxyquinazoline:
[0031] In the first step, add 1.00g of compound 1-trityl-4'-bromomethyl-[1,1]-biphenyl-2-tetrazole and 10mL of tetrahydrofuran into a 25mL double-necked round bottom flask, and stir until The solid was completely dissolved, and 0.72 mL of butylamine was added to react at room temperature for 3 hours, monitored by TLC. After the reaction was completed, the solvent was removed from the obtained solution by rotary evaporation, and the obtained product was dissolved in 25 mL of chloroform. Wash the organic phase (20mL×3) with dilute NaOH solution, anhydrous Na 2 SO 4 Dry and remove solvent in vacuo. Silica gel column chromatography (petroleum ether: ethyl acetate: triethylamine = 2:1:0.01 and ethyl acetate: methanol = 10:1 gradient elution) gave 0.87 g of a colorless viscous solid (88%), It is the product of the first step.
[0032] In the second step, 753 mg (1.37 ...
Embodiment 2
[0034] Example 2: Preparation of 4-{N-butyl-N-[(1H-tetrazol-5-yl]biphenyl)methyl]amine}-6,-hydroxyquinazoline:
[0035] The final product (247mg, 0.53mmol) prepared in Example 1 was added into 1.5mL trimethylsilicon iodide solution, heated to reflux and stirred at room temperature for about 24h. Add excess MeOH solution to the mixture, stir at room temperature for about 12h, after the reaction is complete, spin off the solvent, extract with 30mL of chloroform, take the organic phase with a saturated aqueous solution of sodium bisulfite, pass it through a silica gel column (ethyl acetate:methanol=8:1 ), spin-dried to obtain the target product (147mg, 62%).
Embodiment 3
[0036] Example 3: Preparation of 4-{N-butyl-N-[(1H-tetrazol-5-yl]biphenyl)methyl]amine}-6,7-dimethoxyquinazoline:
[0037]In the second step (the first step is the same as in Example 1 and will not be repeated), take 1.098g (2.0mmol) of the compound 1-trityl-4'-butylaminomethyl-[1,1]-biphenyl- Dissolve 2-tetrazole in 13mL N,N-dimethylformamide, add 0.5mL triethylamine, add 344mg (1.54mmol) 6,7-dimethoxy-4-chloroquinazoline to the mixture, 50°C Under reaction for 96 hours, TLC monitoring, the resulting mixture was added 130mL ethyl acetate, the resulting organic phase was washed with saturated sodium chloride (30ml × 2), anhydrous sodium sulfate, potassium bicarbonate dried, concentrated, passed through a silica gel column (petroleum ether: ethyl acetate = 1:1), rotary evaporation, and drying to obtain 847 mg of white solid, which is the product of the second step, with a yield of 55%.
[0038] In the third step, 2.5 ml of 10% HCl solution was added to the tetrahydrofuran solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com