Variant alpha amylases with enhanced activity on starch polymers
A technology of amylase and starch binding tank, which is applied in the preparation of detergent mixture compositions, detergent compositions, enzymes, etc., and can solve problems such as unsolved accessibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
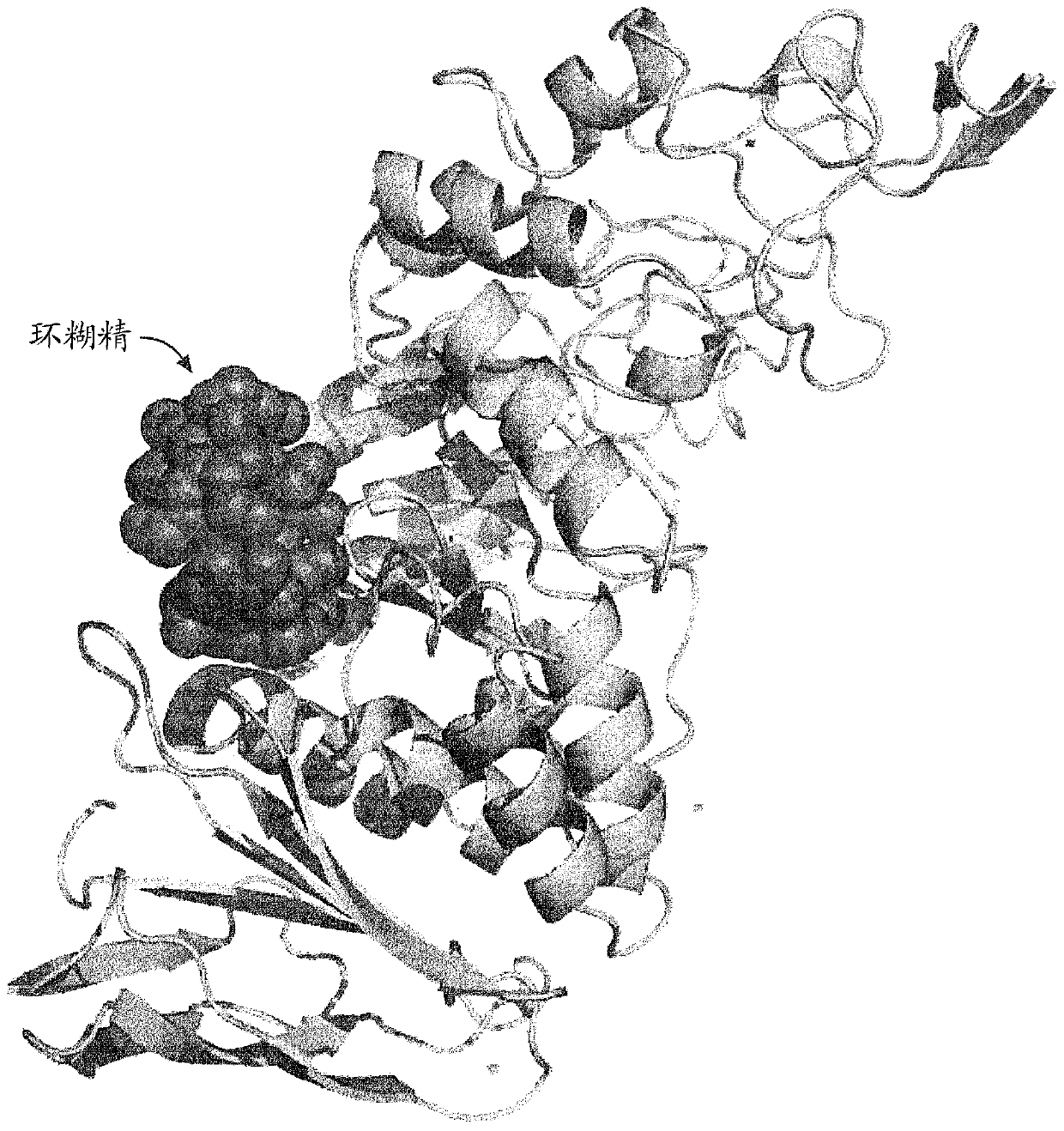
[0259] Example 1: Purification of uPWA
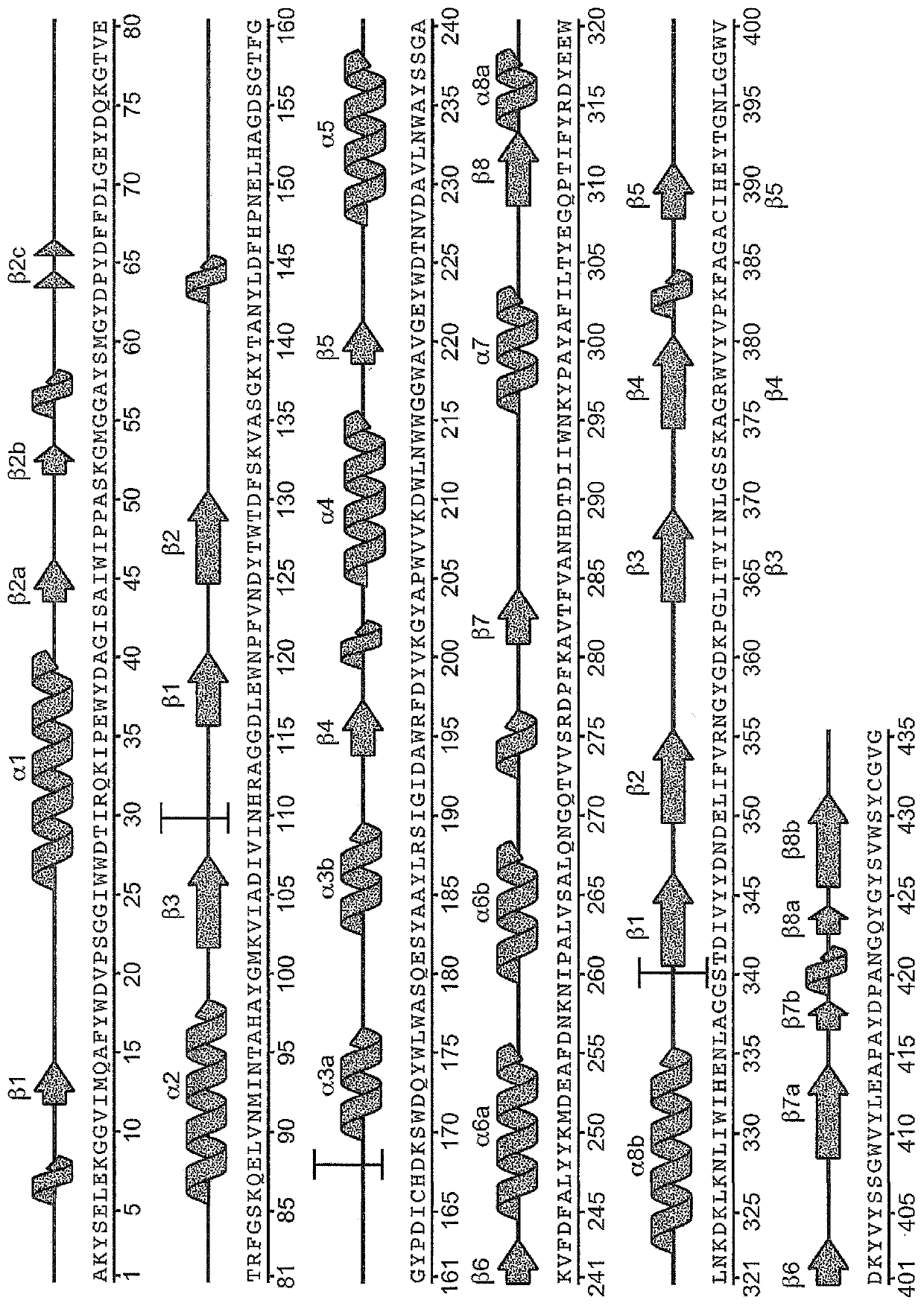
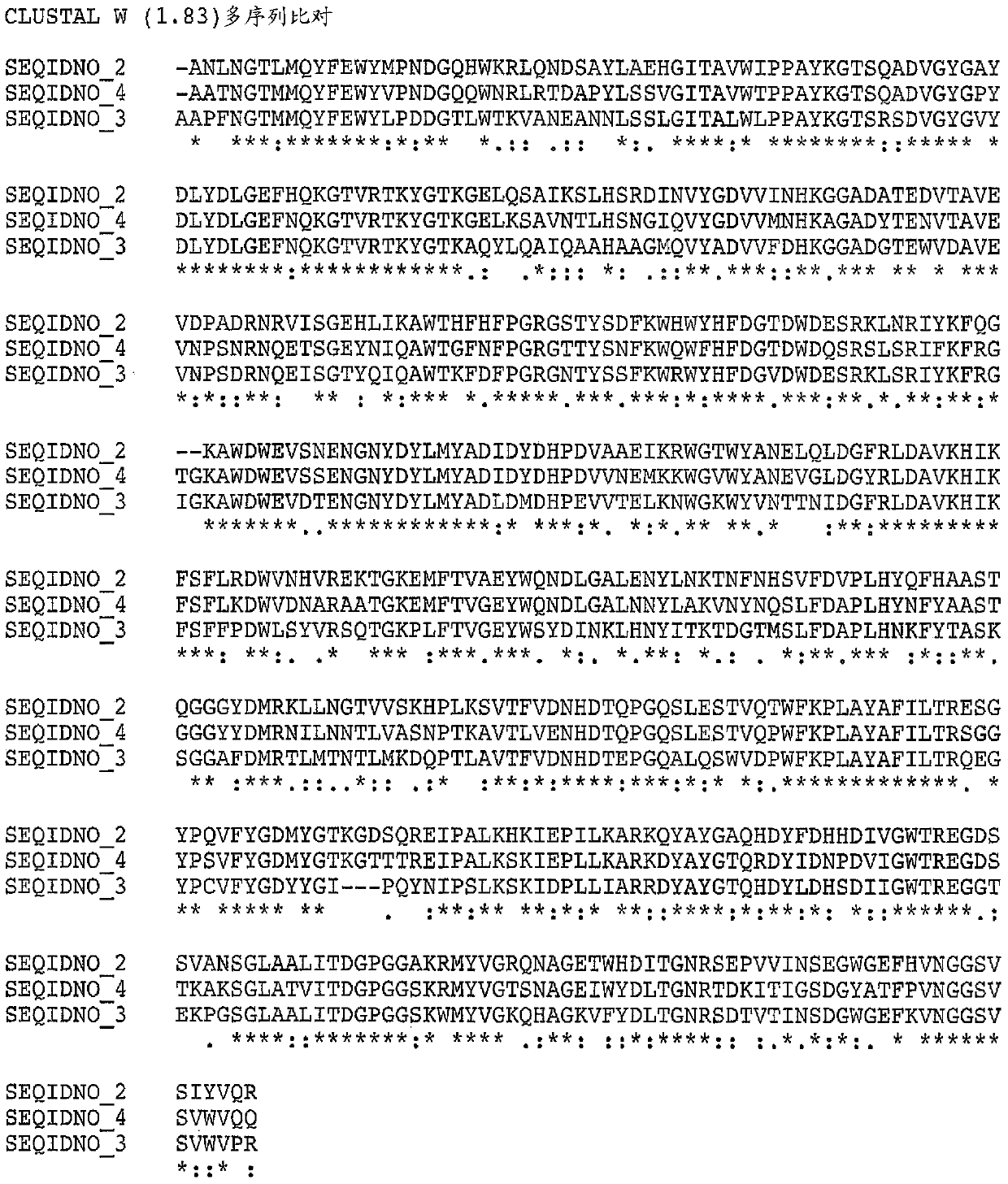
[0260] Variant alpha-amylase uPWA (SEQ ID NO: 2 in US Patent No. 7,273,740) derived from Pyrococcus worriii was purchased from Verenium Corporation (San Diego, CA, USA). uPWA contains 58 amino acid substitutions relative to wild type P. The amino acid sequences of uPWA and PWA are shown in SEQ ID NO:5 and SEQ ID NO:1 below, respectively.
[0261] Variant Pyrococcus worrieli amylase uPWA (SEQ ID NO: 5)
[0262]
[0263] Wild-type Pyrococcus wausii amylase (SEQ ID NO: 1)
[0264]
[0265] The uPWA was purified by hydrophobic interaction chromatography and transferred to a mixture of 50 mM glycine (pH 10) and 2 mM CaCl 2 The composed high pH buffer was then mixed for twenty minutes. Ammonium sulfate was added to achieve a final concentration of 1 M and the solution was mixed for an additional 30 minutes before being applied to a 30 mL phenyl sepharose column equilibrated with the same buffer. The column was washed with the sam...
example 2
[0267] Example 2: Crystallization of uPWA
[0268] Crystallization experiments were performed using 24-well sitting drop in Cryschem plates (Hampton Research) at room temperature. Initial screening was performed using commercially available screening protocols (Hampton and Qiagen). 2 μl of protein sample was mixed with an equal amount of reservoir solution to form a sitting drop and then left to equilibrate against 250 μl of reservoir solution. Crystals appeared after 5-7 days in which stock solution consisted of 0.1M Tris-HCl pH 8.5 and 20% (v / v) ethanol or 0.2M NaCl, 0.1M HEPES pH 7.5 and 10% (v / v) isopropanol composition. Crystals were cryoprotected by adding (dissolving) glucose crystals to sitting drops prior to X-ray diffraction analysis. The crystals were housed in nylon sheaths and quenched in liquid nitrogen.
[0269] Data were collected using synchrotron radiation at the Stanford Synchrotron Radiation Lightsource (SSRL beamline BL12-2). The complete data set w...
example 3
[0270] Example 3. uPWA structure determination and refinement
[0271] The structure of uPWA was determined by molecular replacement using Phaser (McCoy, A.J. et al. (2007) J. Appl. Crystallogr. 40:658-674 (McCoy, A.J. et al., 2007, Journal of Applied Crystallography, pp. 40, pp. 658-674)), wherein the wild-type Pyrococcus wausii amylase (PDB access code: 1MWO; Linden, A. et al. (2003) J. Biol. Chem. 278: 9875-9884 ( The structure of Linden, A. et al., 2003, Journal of Biochemistry, Vol. 278, pp. 9875-9884)) was used as the search model. Using PHENIX (Adams, P.D.et al. (2002) ActaCrystallogr.D Biol.Crystallogr.58:1948-1954 (Adams, P.D. et al., 2002, Acta Crystallographica Series D: Biological Crystallography, Vol. 58, No. 1948-1954 pages)) and COOT (Emsley, P. and Cowtan, K. (2004) ActaCrystallogr.D Biol.Crystallogr.60:2126-2132 (Emsley, P. and Cowtan, K., 2004, Acta Crystallogr. Series D: Biological Crystallography, Volume 60, Pages 2126-2132)) carried out model refinement,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com